Extracorporeal Membrane Oxygenation Characteristics and Outcomes in Children and Adolescents With COVID-19 or Multisystem Inflammatory Syndrome Admitted to U.S. ICUs
- PMID: 36995097
- PMCID: PMC10153593
- DOI: 10.1097/PCC.0000000000003212
Extracorporeal Membrane Oxygenation Characteristics and Outcomes in Children and Adolescents With COVID-19 or Multisystem Inflammatory Syndrome Admitted to U.S. ICUs
Abstract
Objectives: Extracorporeal membrane oxygenation (ECMO) has been used successfully to support adults with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related cardiac or respiratory failure refractory to conventional therapies. Comprehensive reports of children and adolescents with SARS-CoV-2-related ECMO support for conditions, including multisystem inflammatory syndrome in children (MIS-C) and acute COVID-19, are needed.
Design: Case series of patients from the Overcoming COVID-19 public health surveillance registry.
Setting: Sixty-three hospitals in 32 U.S. states reporting to the registry between March 15, 2020, and December 31, 2021.
Patients: Patients less than 21 years admitted to the ICU meeting Centers for Disease Control criteria for MIS-C or acute COVID-19.
Interventions: None.
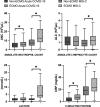
Measurements and main results: The final cohort included 2,733 patients with MIS-C ( n = 1,530; 37 [2.4%] requiring ECMO) or acute COVID-19 ( n = 1,203; 71 [5.9%] requiring ECMO). ECMO patients in both groups were older than those without ECMO support (MIS-C median 15.4 vs 9.9 yr; acute COVID-19 median 15.3 vs 13.6 yr). The body mass index percentile was similar in the MIS-C ECMO versus no ECMO groups (89.9 vs 85.8; p = 0.22) but higher in the COVID-19 ECMO versus no ECMO groups (98.3 vs 96.5; p = 0.03). Patients on ECMO with MIS-C versus COVID-19 were supported more often with venoarterial ECMO (92% vs 41%) for primary cardiac indications (87% vs 23%), had ECMO initiated earlier (median 1 vs 5 d from hospitalization), shorter ECMO courses (median 3.9 vs 14 d), shorter hospital length of stay (median 20 vs 52 d), lower in-hospital mortality (27% vs 37%), and less major morbidity at discharge in survivors (new tracheostomy, oxygen or mechanical ventilation need or neurologic deficit; 0% vs 11%, 0% vs 20%, and 8% vs 15%, respectively). Most patients with MIS-C requiring ECMO support (87%) were admitted during the pre-Delta (variant B.1.617.2) period, while most patients with acute COVID-19 requiring ECMO support (70%) were admitted during the Delta variant period.
Conclusions: ECMO support for SARS-CoV-2-related critical illness was uncommon, but type, initiation, and duration of ECMO use in MIS-C and acute COVID-19 were markedly different. Like pre-pandemic pediatric ECMO cohorts, most patients survived to hospital discharge.
Copyright © 2023 by the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies.
Conflict of interest statement
Dr. Bembea’s institution received funding from the National Institute of Neurological Disorders and Stroke (R01NS106292) and Grifols Investigator Sponsored Research Grant. Drs. Bembea, Heidemann, Zinter, and Randolph received support for article research from the National Institutes of Health (NIH). Dr. Thiagarjan’s institution received funding from the U.S. Department of Defense (Peer Reviewed Medical Research Project Clinical Trial Award No. W81XWH2210301 Trial of Indication-based Transfusion of Red blood cells in Extracorporeal Membrane Oxygenation); he received funding from Society of Critical Care Medicine and the Extracorporeal Life Support Organization. Drs. Young’s, McCadden’s, Newhams’s, Kucuak’s, Mack’s, Fitzgerald’s, Rowan’s, Maddux’s, Kolmar’s, Heidemann’s, Schwartz’s, Kong’s, Crandall’s, Singh’s, Schuster’s, Hall’s, Wellnitz’s, Maamari’s, Gaspers’s, Nofziger’s, Cullimore’s, Halasa’s, McLaughlin’s, Pannaraj’s, Cvijanovich’s, Coates’s, Horwitz’s, Hobbs’s, Dapul’s, and Randolph’s institutions received funding from the U.S. Centers for Disease Control and Prevention (CDC). Dr. McCadden disclosed work for hire. Dr. Newhams’ institution received funding from the National Institute of Allergy and Infectious Diseases. Drs. Fitzgerald’s, Kong’s, Cullimore’s, Cvijanovich’s, and Randolph’s institutions received funding from the NIH. Dr. Rowan’s institution received funding from the National Heart, Lung, and Blood Institute (NHLBI) (K23HL150244). Dr. Maddux’s institution received funding from the National Institute of Child Health and Human Development (K23HD096018). Drs. Irby, Crandall, Singh, Wellnitz, Nofziger, Bradford, McLaughlin, Coates, Hobbs, and Zambrano received support for article research from the CDC. Dr. Schuster’s institution received funding from Merck. Dr. Hall received funding from Abbvie, Kiadis, and the American Board of Pediatrics. Dr. Wellnitz’s institution received funding from the University of Pennsylvania (prime sponsor NIH) and the University of Nebraska (prime sponsor Administration for Strategic Preparedness and Response). Dr. Gaspers received funding from Abbott Laboratories. Dr. Munoz’s institution received funding from Boston’s Children’s Hospital; he received funding from the University of Texas Health Science Center at Houston. Dr. Halasa’s institution received funding from Sanofi. Dr. McLaughlin received funding from expert witness fees from two entities. Dr. Pannaraj’s institution received funding from AstraZeneca and Pfizer. Dr. Coates’ institution received funding from the NHLBI and the American Lung Association; she received funding from Sobi. Drs. Hobbs and Randolph received funding from UpToDate. Dr. Hobbs received funding from Dynamed.com; she disclosed that she was a speaker for Biomerieux 2021–2022. Drs. Zambrano and Campbell disclosed government work. Dr. Randolph had full access to all the data in the investigation and takes responsibility for the integrity of the data and the accuracy of the data analysis. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures
Comment in
-
Tickle Me ECMO…Differences and Outcomes Unearthed for Kids Requiring Extracorporeal Membrane Oxygenation in Severe Acute Respiratory Syndrome Coronavirus 2-Associated Disease.Pediatr Crit Care Med. 2023 May 1;24(5):419-422. doi: 10.1097/PCC.0000000000003225. Epub 2023 May 4. Pediatr Crit Care Med. 2023. PMID: 37140332 Free PMC article. No abstract available.
References
-
- Castagnoli R, Votto M, Licari A, et al. : Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatr 2020; 174:882–889 - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

