Absorption, Metabolism, and Excretion of [14C]-Tebipenem Pivoxil Hydrobromide (TBP-PI-HBr) in Healthy Male Subjects
- PMID: 36995239
- PMCID: PMC10112213
- DOI: 10.1128/aac.01509-22
Absorption, Metabolism, and Excretion of [14C]-Tebipenem Pivoxil Hydrobromide (TBP-PI-HBr) in Healthy Male Subjects
Abstract
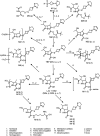
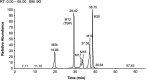
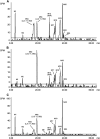
Tebipenem pivoxil hydrobromide (TBP-PI-HBr) is an oral prodrug of pharmacologically active moiety tebipenem (TBP), which is a carbapenem with activity against multidrug-resistant Gram-negative pathogens. Conversion from the prodrug to the active moiety, namely, TBP, occurs in the enterocytes of the gastrointestinal tract via intestinal esterases. The absorption, metabolism, and excretion in humans were evaluated, following the administration of a single oral dose of [14C]-TBP-PI-HBr. Healthy male subjects (n = 8) received a single 600 mg oral dose of TBP-PI-HBr containing approximately 150 μCi of [14C]-TBP-PI-HBr. Blood, urine, and fecal samples were collected to determine the total radioactivity, concentrations of TBP (plasma only), and metabolite profiling and identification. The overall mean recovery of the total radioactivity in urine (38.7%) and feces (44.6%) combined was approximately 83.3% of the administered dose, with individual recoveries ranging from 80.1% to 85.0%. Plasma TBP LC-MS/MS and metabolite profiling data suggest that TBP was the main circulating component in plasma and that it accounts for approximately 54% of the total plasma radioactivity, based on the plasma AUC ratio of TBP/total radioactivity. The ring-open metabolite LJC 11562 was another major component in plasma (>10%). TBP (M12), LJC 11562, and four trace to minor metabolites were identified/characterized in the urine. TBP-PI, TBP (M12), and 11 trace to minor metabolites were identified/characterized in the feces. The renal and fecal routes are major clearance pathways in the elimination of [14C]-TBP-PI-HBr, with a mean combined recovery of 83.3%. TBP and its inactive ring-open metabolite LJC 11562 were the major circulating metabolites in the plasma.
Keywords: absorption; excretion; mass balance; metabolism; tebipenem.
Conflict of interest statement
The authors declare a conflict of interest. G.M. and L.G. are consultants to Spero Therapeutics, Inc. All other authors were paid employees of Spero Therapeutics, Cambridge, MA at the time of this study.
Figures



References
-
- Citron DM, Tyrrell KL, Rubio A, Goldstein EJC. 2018. In vitro activity of tebipenem (SPR859), tebipenem-pivoxil (SPR994) and meropenem against a broad spectrum of anaerobic bacteria. SUN-559. ASM Microbe.
-
- Mendes RE, Rhomberg PR, Watters A, Cotroneo N, Rubio A, Flamm RK. 2018. Antimicrobial activity assessment of tebipenem (SPR859) against an isolate collection causing urinary tract infections. ASM Microbe.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

