Phagocytosis increases an oxidative metabolic and immune suppressive signature in tumor macrophages
- PMID: 36995340
- PMCID: PMC10067971
- DOI: 10.1084/jem.20221472
Phagocytosis increases an oxidative metabolic and immune suppressive signature in tumor macrophages
Abstract
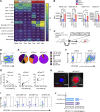
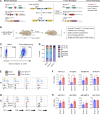
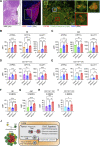
Phagocytosis is a key macrophage function, but how phagocytosis shapes tumor-associated macrophage (TAM) phenotypes and heterogeneity in solid tumors remains unclear. Here, we utilized both syngeneic and novel autochthonous lung tumor models in which neoplastic cells express the fluorophore tdTomato (tdTom) to identify TAMs that have phagocytosed neoplastic cells in vivo. Phagocytic tdTompos TAMs upregulated antigen presentation and anti-inflammatory proteins, but downregulated classic proinflammatory effectors compared to tdTomneg TAMs. Single-cell transcriptomic profiling identified TAM subset-specific and common gene expression changes associated with phagocytosis. We uncover a phagocytic signature that is predominated by oxidative phosphorylation (OXPHOS), ribosomal, and metabolic genes, and this signature correlates with worse clinical outcome in human lung cancer. Expression of OXPHOS proteins, mitochondrial content, and functional utilization of OXPHOS were increased in tdTompos TAMs. tdTompos tumor dendritic cells also display similar metabolic changes. Our identification of phagocytic TAMs as a distinct myeloid cell state links phagocytosis of neoplastic cells in vivo with OXPHOS and tumor-promoting phenotypes.
© 2023 Gonzalez et al.
Conflict of interest statement
Disclosures: M.A. Gonzalez is a current employee of Gilead Sciences. D.R. Lu reported currently being an employee of Amgen, Inc. M. Yousefi reported other from Pionyr Immunotherapeutics outside the submitted work. C.G. Briseño reported other from Amgen, Inc. during the conduct of the study; other from Amgen, Inc. outside the submitted work; and being an employee of Amgen, Inc. J.E.V. Watson reported being an employee of Amgen, Inc. and holding Amgen, Inc. stock. V. Arias reported currently being employed at Amgen, Inc. C.M. Li reported non-financial support from Amgen, Inc. during the conduct of the study; and personal fees from Amgen, Inc. outside the submitted work. M.M. Winslow reported grants from NIH during the conduct of the study; and is a cofounder, advisor, and has equity in D2G Oncology, Inc. K.V. Tarbell reported personal fees from Amgen, Inc. during the conduct of the study; personal fees from Amgen, Inc. outside the submitted work; and being an employee of Amgen, Inc. In addition, Amgen, Inc. financially supported the research included in this manuscript. No other disclosures were reported.
Figures











References
-
- A-Gonzalez, N., Bensinger S.J., Hong C., Beceiro S., Bradley M.N., Zelcer N., Deniz J., Ramirez C., Díaz M., Gallardo G., et al. 2009. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 31:245–258. 10.1016/j.immuni.2009.06.018 - DOI - PMC - PubMed
-
- A-Gonzalez, N., Quintana J.A., García-Silva S., Mazariegos M., González de la Aleja A., Nicolás-Ávila J.A., Walter W., Adrover J.M., Crainiciuc G., Kuchroo V.K., et al. 2017. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 214:1281–1296. 10.1084/jem.20161375 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

