Standardizing lymphangiography and lymphatic interventions: a preclinical in vivo approach with detailed procedural steps
- PMID: 36995443
- PMCID: PMC10063775
- DOI: 10.1186/s42155-023-00364-z
Standardizing lymphangiography and lymphatic interventions: a preclinical in vivo approach with detailed procedural steps
Abstract
Purpose: To present a preclinical in vivo approach for standardization and training of lymphangiography and lymphatic interventions using a pictorial review.
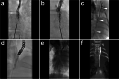
Materials and methods: Different lipiodol- and gadolinium-based lymphangiography and lymphatic interventions were performed in twelve (12) landrace pigs with a mean bodyweight of 34 ± 2 kg using various imaging and guiding modalities, similar to the procedures used in humans. The techniques used were explicitly introduced and illustrated. The potential applications of each technique in preclinical training were also discussed.
Results: By applying visual, ultrasonography, fluoroscopy, CT, cone-beam CT, and/or MRI examination or guidance, a total of eleven techniques were successfully implemented in twelve pigs. The presented techniques include inguinal postoperative lymphatic leakage (PLL) establishment, interstitial dye test, five types of lymphangiography [incl. lipiodol-based translymphatic lymphangiography (TL), lipiodol-based percutaneous intranodal lymphangiography (INL), lipiodol-based laparotomic INL, lipiodol-based interstitial lymphangiography, and interstitial magnetic resonance lymphangiography (MRL)], and four types of percutaneous interventions in the treatment of PLL [incl. thoracic duct embolization (TDE), intranodal embolization (INE), afferent lymphatic vessel sclerotherapy (ALVS), and afferent lymphatic vessel embolization (ALVE)].
Conclusion: This study provides a valuable resource for inexperienced interventional radiologists to undergo the preclinical training in lymphangiography and lymphatic interventions using healthy pig models.
Keywords: Animal Model; Lymphatic Intervention; Lymphography; Postoperative Lymphatic Leakage; Preclinical Training.
© 2023. The Author(s).
Conflict of interest statement
The corresponding author declares that this animal study was financially supported by Research & Innovation Department, Guerbet, Roissy, France.
Figures












References
-
- Baek Y, Won JH, Kong TW, Paek J, Chang SJ, Ryu HS, Kim J. Lymphatic Leak Occurring After Surgical Lymph Node Dissection: A Preliminary Study Assessing the Feasibility and Outcome of Lymphatic Embolization. Cardiovasc Intervent Radiol. 2016;39(12):1728–1735. doi: 10.1007/s00270-016-1435-x. - DOI - PubMed
-
- Dargent M, Chassard JL, Pommateau E, Samim F. Failures and complications of lymphography with ultrafluid lipiodol. Lyon Chir. 1962;58:870–876. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
