Complement C3a treatment accelerates recovery after stroke via modulation of astrocyte reactivity and cortical connectivity
- PMID: 36995772
- PMCID: PMC10178843
- DOI: 10.1172/JCI162253
Complement C3a treatment accelerates recovery after stroke via modulation of astrocyte reactivity and cortical connectivity
Abstract
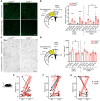
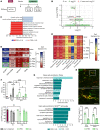
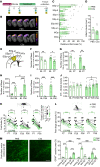
Despite advances in acute care, ischemic stroke remains a major cause of long-term disability. Approaches targeting both neuronal and glial responses are needed to enhance recovery and improve long-term outcome. The complement C3a receptor (C3aR) is a regulator of inflammation with roles in neurodevelopment, neural plasticity, and neurodegeneration. Using mice lacking C3aR (C3aR-/-) and mice overexpressing C3a in the brain, we uncovered 2 opposing effects of C3aR signaling on functional recovery after ischemic stroke: inhibition in the acute phase and facilitation in the later phase. Peri-infarct astrocyte reactivity was increased and density of microglia reduced in C3aR-/- mice; C3a overexpression led to the opposite effects. Pharmacological treatment of wild-type mice with intranasal C3a starting 7 days after stroke accelerated recovery of motor function and attenuated astrocyte reactivity without enhancing microgliosis. C3a treatment stimulated global white matter reorganization, increased peri-infarct structural connectivity, and upregulated Igf1 and Thbs4 in the peri-infarct cortex. Thus, C3a treatment from day 7 after stroke exerts positive effects on astrocytes and neuronal connectivity while avoiding the deleterious consequences of C3aR signaling during the acute phase. Intranasal administration of C3aR agonists within a convenient time window holds translational promise to improve outcome after ischemic stroke.
Keywords: Complement; Neuroscience; Stroke.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

