Modulating fast skeletal muscle contraction protects skeletal muscle in animal models of Duchenne muscular dystrophy
- PMID: 36995778
- PMCID: PMC10178848
- DOI: 10.1172/JCI153837
Modulating fast skeletal muscle contraction protects skeletal muscle in animal models of Duchenne muscular dystrophy
Abstract
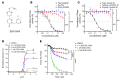
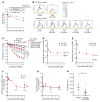
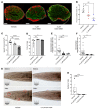
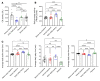
Duchenne muscular dystrophy (DMD) is a lethal muscle disease caused by absence of the protein dystrophin, which acts as a structural link between the basal lamina and contractile machinery to stabilize muscle membranes in response to mechanical stress. In DMD, mechanical stress leads to exaggerated membrane injury and fiber breakdown, with fast fibers being the most susceptible to damage. A major contributor to this injury is muscle contraction, controlled by the motor protein myosin. However, how muscle contraction and fast muscle fiber damage contribute to the pathophysiology of DMD has not been well characterized. We explored the role of fast skeletal muscle contraction in DMD with a potentially novel, selective, orally active inhibitor of fast skeletal muscle myosin, EDG-5506. Surprisingly, even modest decreases of contraction (<15%) were sufficient to protect skeletal muscles in dystrophic mdx mice from stress injury. Longer-term treatment also decreased muscle fibrosis in key disease-implicated tissues. Importantly, therapeutic levels of myosin inhibition with EDG-5506 did not detrimentally affect strength or coordination. Finally, in dystrophic dogs, EDG-5506 reversibly reduced circulating muscle injury biomarkers and increased habitual activity. This unexpected biology may represent an important alternative treatment strategy for Duchenne and related myopathies.
Keywords: Muscle Biology; Neuromuscular disease; Skeletal muscle; Therapeutics.
Conflict of interest statement
Figures


References
-
- Petrof BJ. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Mol Cell Biochem. 1998;179(1–2):111–123. - PubMed

