The chemical ecology of coumarins and phenazines affects iron acquisition by pseudomonads
- PMID: 36996105
- PMCID: PMC10083548
- DOI: 10.1073/pnas.2217951120
The chemical ecology of coumarins and phenazines affects iron acquisition by pseudomonads
Abstract
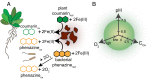
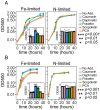
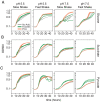
Secondary metabolites are important facilitators of plant-microbe interactions in the rhizosphere, contributing to communication, competition, and nutrient acquisition. However, at first glance, the rhizosphere seems full of metabolites with overlapping functions, and we have a limited understanding of basic principles governing metabolite use. Increasing access to the essential nutrient iron is one important, but seemingly redundant role performed by both plant and microbial Redox-Active Metabolites (RAMs). We used coumarins, RAMs made by the model plant Arabidopsis thaliana, and phenazines, RAMs made by soil-dwelling pseudomonads, to ask whether plant and microbial RAMs might each have distinct functions under different environmental conditions. We show that variations in oxygen and pH lead to predictable differences in the capacity of coumarins vs phenazines to increase the growth of iron-limited pseudomonads and that these effects depend on whether pseudomonads are grown on glucose, succinate, or pyruvate: carbon sources commonly found in root exudates. Our results are explained by the chemical reactivities of these metabolites and the redox state of phenazines as altered by microbial metabolism. This work shows that variations in the chemical microenvironment can profoundly affect secondary metabolite function and suggests plants may tune the utility of microbial secondary metabolites by altering the carbon released in root exudates. Together, these findings suggest that RAM diversity may be less overwhelming when viewed through a chemical ecological lens: Distinct molecules can be expected to be more or less important to certain ecosystem functions, such as iron acquisition, depending on the local chemical microenvironments in which they reside.
Keywords: coumarin; phenazine; pseudomonas; redox; secondary metabolite.
Conflict of interest statement
The authors declare no competing interest.
Figures


Comment in
-
Chemical symphony of coumarins and phenazines in rhizosphere iron solubilization.Proc Natl Acad Sci U S A. 2023 May 2;120(18):e2304171120. doi: 10.1073/pnas.2304171120. Epub 2023 Apr 24. Proc Natl Acad Sci U S A. 2023. PMID: 37094125 Free PMC article. No abstract available.
Similar articles
-
The phospho-ferrozine assay: a tool to study bacterial redox-active metabolites produced at the plant root.Appl Environ Microbiol. 2025 Jan 31;91(1):e0219424. doi: 10.1128/aem.02194-24. Epub 2024 Dec 17. Appl Environ Microbiol. 2025. PMID: 39688434 Free PMC article.
-
Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome.Proc Natl Acad Sci U S A. 2019 Jun 18;116(25):12558-12565. doi: 10.1073/pnas.1820691116. Epub 2019 May 31. Proc Natl Acad Sci U S A. 2019. PMID: 31152139 Free PMC article.
-
Feruloyl-CoA 6'-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis.Plant Physiol. 2014 Jan;164(1):160-72. doi: 10.1104/pp.113.228544. Epub 2013 Nov 18. Plant Physiol. 2014. PMID: 24246380 Free PMC article.
-
Mobilization of Iron by Plant-Borne Coumarins.Trends Plant Sci. 2017 Jun;22(6):538-548. doi: 10.1016/j.tplants.2017.03.008. Epub 2017 Apr 3. Trends Plant Sci. 2017. PMID: 28385337 Review.
-
Tapping into Plant-Microbiome Interactions through the Lens of Multi-Omics Techniques.Cells. 2022 Oct 17;11(20):3254. doi: 10.3390/cells11203254. Cells. 2022. PMID: 36291121 Free PMC article. Review.
Cited by
-
The phospho-ferrozine assay: a tool to study bacterial redox-active metabolites produced at the plant root.Appl Environ Microbiol. 2025 Jan 31;91(1):e0219424. doi: 10.1128/aem.02194-24. Epub 2024 Dec 17. Appl Environ Microbiol. 2025. PMID: 39688434 Free PMC article.
-
Gut microbiomes of cycad-feeding insects tolerant to β-methylamino-L-alanine (BMAA) are rich in siderophore biosynthesis.ISME Commun. 2023 Nov 22;3(1):122. doi: 10.1038/s43705-023-00323-8. ISME Commun. 2023. PMID: 37993724 Free PMC article.
-
Unraveling plant-microbe interactions: can integrated omics approaches offer concrete answers?J Exp Bot. 2024 Feb 28;75(5):1289-1313. doi: 10.1093/jxb/erad448. J Exp Bot. 2024. PMID: 37950741 Free PMC article.
-
Surface-active antibiotic production as a multifunctional adaptation for postfire microorganisms.ISME J. 2024 Jan 8;18(1):wrae022. doi: 10.1093/ismejo/wrae022. ISME J. 2024. PMID: 38366029 Free PMC article.
-
Rice Varieties Intercropping Induced Soil Metabolic and Microbial Recruiting to Enhance the Rice Blast (Magnaporthe Oryzae) Resistance.Metabolites. 2024 Sep 20;14(9):507. doi: 10.3390/metabo14090507. Metabolites. 2024. PMID: 39330514 Free PMC article.
References
-
- Lebeis S. L., et al. , Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 349, 860–864 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

