Cytosolic antibody receptor TRIM21 is required for effective tau immunotherapy in mouse models
- PMID: 36996217
- PMCID: PMC7614512
- DOI: 10.1126/science.abn1366
Cytosolic antibody receptor TRIM21 is required for effective tau immunotherapy in mouse models
Abstract
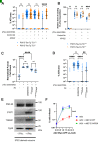
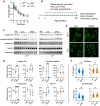
Aggregates of the protein tau are proposed to drive pathogenesis in neurodegenerative diseases. Tau can be targeted by using passively transferred antibodies (Abs), but the mechanisms of Ab protection are incompletely understood. In this work, we used a variety of cell and animal model systems and showed that the cytosolic Ab receptor and E3 ligase TRIM21 (T21) could play a role in Ab protection against tau pathology. Tau-Ab complexes were internalized to the cytosol of neurons, which enabled T21 engagement and protection against seeded aggregation. Ab-mediated protection against tau pathology was lost in mice that lacked T21. Thus, the cytosolic compartment provides a site of immunotherapeutic protection, which may help in the design of Ab-based therapies in neurodegenerative disease.
Conflict of interest statement
WAM, LCJ, ASM, LVCM, SK, AES, and BJT are listed as inventors on a patent related to this study. WAM has acted as consultant to Ahren Innovation Capital.
Figures


References
-
- Goedert M. Tau filaments in neurodegenerative diseases. FEBS Lett. 2018;592:2383–239. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

