Intracellular cGMP increase is not involved in thyroid cancer cell death
- PMID: 36996255
- PMCID: PMC10062617
- DOI: 10.1371/journal.pone.0283888
Intracellular cGMP increase is not involved in thyroid cancer cell death
Abstract
Introduction: Type 5 phosphodiesterase (PDE5) inhibitors (PDE5i) lead to intracellular cyclic-guanosine monophosphate (cGMP) increase and are used for clinical treatment of erectile dysfunction. Studies found that cGMP may up/downregulate the growth of certain endocrine tumor cells, suggesting that PDE5i could impact cancer risk.
Aim: We evaluated if PDE5i may modulate thyroid cancer cell growth in vitro.
Materials and methods: We used malignant (K1) and benign (Nthy-ori 3-1) thyroid cell lines, as well as the COS7 cells as a reference model. Cells were treated 0-24 h with the PDE5i vardenafil or the cGMP analog 8-br-cGMP (nM-μM range). cGMP levels and caspase 3 cleavage were evaluated by BRET, in cGMP or caspase 3 biosensor-expressing cells. Phosphorylation of the proliferation-associated extracellularly-regulated kinases 1 and 2 (ERK1/2) was evaluated by Western blotting, while nuclear fragmentation by DAPI staining. Cell viability was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
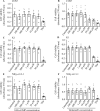
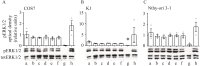
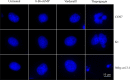
Results: Both vardenafil and 8-br-cGMP effectively induced dose-dependent cGMP BRET signals (p≤0.05) in all the cell lines. However, no differences in caspase 3 activation occurred comparing PDE5i-treated vs untreated cells, at all concentrations and time-points tested (p>0.05). These results match those obtained upon cell treatment with 8-br-cGMP, which failed in inducing caspase 3 cleavage in all the cell lines (p>0.05). Moreover, they reflect the lack of nuclear fragmentation. Interestingly, the modulation of intracellular cGMP levels with vardenafil or the analog did not impact cell viability of both malignant and benign thyroid tumor cell lines, nor the phosphorylation of ERK1/2 (p>0.05).
Conclusions: This study demonstrates that increased cGMP levels are not linked to cell viability or death in K1 and Nthy-ori 3-1 cell lines, suggesting that PDE5i do not impact the growth of thyroid cancer cells. Since different results were previously published, further investigations are recommended to clarify the impact of PDE5i on thyroid cancer cells.
Copyright: © 2023 Alessandro et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

