A new multiplex SARS-CoV-2 antigen microarray showed correlation of IgG, IgA, and IgM antibodies from patients with COVID-19 disease severity and maintenance of relative IgA and IgM antigen binding over time
- PMID: 36996259
- PMCID: PMC10062637
- DOI: 10.1371/journal.pone.0283537
A new multiplex SARS-CoV-2 antigen microarray showed correlation of IgG, IgA, and IgM antibodies from patients with COVID-19 disease severity and maintenance of relative IgA and IgM antigen binding over time
Abstract
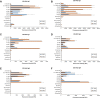
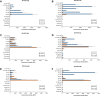
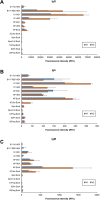
Zoonotic spillover of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to humans in December 2019 caused the coronavirus disease 2019 (COVID-19) pandemic. Serological monitoring is critical for detailed understanding of individual immune responses to infection and protection to guide clinical therapeutic and vaccine strategies. We developed a high throughput multiplexed SARS-CoV-2 antigen microarray incorporating spike (S) and nucleocapsid protein (NP) and fragments expressed in various hosts which allowed simultaneous assessment of serum IgG, IgA, and IgM responses. Antigen glycosylation influenced antibody binding, with S glycosylation generally increasing and NP glycosylation decreasing binding. Purified antibody isotypes demonstrated a binding pattern and intensity different from the same isotype in whole serum, probably due to competition from the other isotypes present. Using purified antibody isotypes from naïve Irish COVID-19 patients, we correlated antibody isotype binding to different panels of antigens with disease severity, with binding to the S region S1 expressed in insect cells (S1 Sf21) significant for IgG, IgA, and IgM. Assessing longitudinal response for constant concentrations of purified antibody isotypes for a patient subset demonstrated that the relative proportion of antigen-specific IgGs decreased over time for severe disease, but the relative proportion of antigen-specific IgA binding remained at the same magnitude at 5 and 9 months post-first symptom onset. Further, the relative proportion of IgM binding decreased for S antigens but remained the same for NP antigens. This may support antigen-specific serum IgA and IgM playing a role in maintaining longer-term protection, important for developing and assessing vaccine strategies. Overall, these data demonstrate the multiplexed platform as a sensitive and useful platform for expanded humoral immunity studies, allowing detailed elucidation of antibody isotypes response against multiple antigens. This approach will be useful for monoclonal antibody therapeutic studies and screening of donor polyclonal antibodies for patient infusions.
Copyright: © 2023 Berre et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- World Health Organisation. WHO Coronavirus (COVID-19) Dashboard. 2022; Available from: https://covid19.who.int/.
-
- Peghin M, Martino MD, Fabris M, Palese A, Visintini E, Graziano E, et al.. The fall in antibody response to SARS-CoV-2: a longitudinal study of asymptomatic to critically ill patients up to 10 months after recovery. Journal of Clinical Microbiology. 2021; 59(11): e01138–21. 10.1128/JCM.01138-21 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

