Allyl Alcohol as an Acrolein Equivalent in Enantioselective C-C Coupling: Total Synthesis of Amphidinolides R, J, and S
- PMID: 36996284
- PMCID: PMC10101927
- DOI: 10.1021/jacs.3c01809
Allyl Alcohol as an Acrolein Equivalent in Enantioselective C-C Coupling: Total Synthesis of Amphidinolides R, J, and S
Abstract
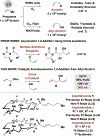
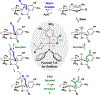
The first systematic study of catalytic enantioselective 1,2-additions to acrolein is described. Specifically, using allyl alcohol as a tractable, inexpensive acrolein proelectrophile, iridium-catalyzed acrolein allylation is achieved with high levels of regio-, anti-diastereo-, and enantioselectivity. This process delivers 3-hydroxy-1,5-hexadienes, a useful compound class that is otherwise challenging to access via enantioselective catalysis. Two-fold use of this method unlocks concise total syntheses of amphidinolide R (9 vs 23 steps, LLS) and amphidinolide J (9 vs 23 or 26 steps, LLS), which are prepared in fewer than half the steps previously possible, and the first total synthesis of amphidinolide S (10 steps, LLS).
Conflict of interest statement
The authors declare no competing financial interest.
Figures
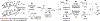

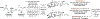
References
-
- Arntz D; Fischer A; Hopp M; Jacobi S; Sauer J; Ohara T; Sat T; Shimizu N; Schwind H Acrolein and Methacrolein. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VHC: Weinheim, Germany, 2007; Vol. 1, pp 329–346.
-
- The two largest volume industrial processes using acrolein are the synthesis of acrylic acid and DL-methionine. See:
- Sun D; Yamada Y; Sato S; Ueda W Glycerol Hydrogenolysis into Useful C3 Chemicals. Appl. Catal. B 2016, 193, 75–92.
- Sanders JPM; Sheldon RA Comparison of the Sustainability Metrics of the Petrochemical and Biomass-Based Routes to Methionine. Catal. Today 2015, 239, 44–49.
-
- For reviews describing use of acrolein as a biocide, see:
- Izard C; Libermann C Acrolein. Mut. Res 1978, 47, 115–138. - PubMed
- Faroon O; Roney N; Tayler J; Ashizawa A; Lumpkin MH; Plewak DJ Acrolein Environmental Levels and Potential for Human Exposure. Toxicol. Ind. Health 2008, 24, 543–564. - PubMed
- See also ref 1.
-
- For reviews on the health effects and regulation of acrolein, see:
- Ghilarducci DP; Tieerdema RS Fate and Effects of Acrolein. In Reviews of Environmental Contamination and Toxicology; Springer-Verlag, New York, 1995; Vol 144. pp 95–146. - PubMed
- Gomes R; Meek ME World Health Organization & International Programme on Chemical Safety: Acrolein. 2002, 1–49.
- Environmental Protection Agency (EPA). Title 40, Chapter I, Subchapter J, Part 302. Designation, Reportable Quantities, and Notification. Code of Federal Regulations 2008, pp 5–509.
- Mgohe A; Smita G; Lamoreau B; Mohammad M; Barve S; McClain C; Joshi-Barve S Molecular Mechanisms of Acrolein Toxicity: Relevance to Human Disease. Toxicol. Sci 2015, 143, 242–255. - PMC - PubMed
- Henning RJ; Johnson GT; Coyle JP; Harbison RD Acrolein Can Cause Cardiovascular Disease: A Review. Cardiovasc. Toxicol 2017, 17, 227–236. - PubMed
-
- For selected examples of the use of chiral auxiliaries in asymmetric 1,2-additions to acrolein, see:
- Evans DA; Gage JR; Leighton JL Total Synthesis of (+)-Calyculin A. J. Am. Chem. Soc 1992, 114, 9434–9453.
- Hirose T; Sunazauka T; Yamamoto D; Kojima N Tatsuya S; Yoshihiro H; Kuwajima I; Omura S Determination of the Absolute Stereochemistry and Asymmetric Total Synthesis of Madindolines A and B: A Practical Improvement to a Second-Generation Approach from the First-Generation. Tetrahedron 2005, 61, 6015–6039.
- Crimmins MT; Zhang Y; Diaz FA Total Synthesis of (−)-Mucocin. Org. Lett 2006, 8, 2369–2372. - PMC - PubMed
- Gebaur J; Arseniyadis S; Cossy J A Concise Total Synthesis of Melithiazole C. Org. Lett 2007, 9, 3425–3427. - PubMed
- Lee H; Kim KW; Park J; Kim H; Kim S A General Strategy for Construction of Both 2,6-Cis and 2,6-Trans-Disubstituted Tetrahydropurans: Substrate-Controlled Asymmetric Total Synthesis of (+)-Scanlonenyne. Angew. Chem. Int. Ed 2008, 47, 4200–4203. - PubMed
- Calo F; Richardson J; Barrett AGM Total Synthesis of Citrafungin A. J. Org. Chem 2008, 73, 9692–9697. - PubMed
- Crimmins MT; Ellis JM; Emmitte KA; Haile PA; McDougall PJ; Parrish JD; Zuccarello JL Enantioselective Total Synthesis of Brevetoxin A: Unified Strategy for the B, E, G, and J Subunits. Chem. Eur. J 2009, 15, 9223–9234. - PMC - PubMed
- Cook C; Guinchard X; Liron F; Roulland E Total Synthesis of (−)-Exiguolide. Org. Lett 2010, 12, 744–747. - PubMed
- Colon A; Hoffman TJ; Gebauer J; Dash J; Rigby JH; Arseniyadis S; Cossy J Catalysis-Based Enantioselective Total Synthesis of Myxothiazole Z, (14S)-Melithiazole G, and (14S)-Cystothiazole F. Chem. Commun 2012, 48, 10508–10510. - PubMed
- Kobayashi T; Tanaka K; Ishida M; Yamakita N; Abe H; Ito H Asymmetric Total Synthesis of Pleurospiroketals A and B. Chem. Commun 2018, 54, 10316–10319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

