Tricarbonyl-Pyrazine-Molybdenum(0) Metal-Organic Frameworks for the Storage and Delivery of Biologically Active Carbon Monoxide
- PMID: 36996427
- PMCID: PMC10091354
- DOI: 10.1021/acsbiomaterials.3c00140
Tricarbonyl-Pyrazine-Molybdenum(0) Metal-Organic Frameworks for the Storage and Delivery of Biologically Active Carbon Monoxide
Abstract
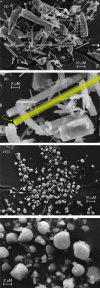
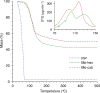
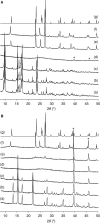
Metal-organic frameworks (MOFs) have high potential as nanoplatforms for the storage and delivery of therapeutic gasotransmitters or gas-releasing molecules. The aim of the present study was to open an investigation into the viability of tricarbonyl-pyrazine-molybdenum(0) MOFs as carbon monoxide-releasing materials (CORMAs). A previous investigation found that the reaction of Mo(CO)6 with excess pyrazine (pyz) in a sealed ampoule gave a mixture comprising a major triclinic phase with pyz-occupied hexagonal channels, formulated as fac-Mo(CO)3(pyz)3/2·1/2pyz (Mo-hex), and a minor dense cubic phase, formulated as fac-Mo(CO)3(pyz)3/2 (Mo-cub). In the present work, an open reflux method in toluene has been optimized for the large-scale synthesis of the pure Mo-cub phase. The crystalline solids Mo-hex and Mo-cub were characterized by powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), FT-IR and FT-Raman spectroscopies, and 13C{1H} cross-polarization (CP) magic-angle spinning (MAS) NMR spectroscopy. The release of CO from the MOFs was studied by the deoxy-myoglobin (deoxy-Mb)/carbonmonoxy-myoglobin (MbCO) UV-vis assay. Mo-hex and Mo-cub release CO upon contact with a physiological buffer in the dark, delivering 0.35 and 0.22 equiv (based on Mo), respectively, after 24 h, with half-lives of 3-4 h. Both materials display high photostability such that the CO-releasing kinetics is not affected by irradiation of the materials with UV light. These materials are attractive as potential CORMAs due to the slow release of a high CO payload. In the solid-state and under open air, Mo-cub underwent almost complete decarbonylation over a period of 4 days, corresponding to a theoretical CO release of 10 mmol per gram of material.
Keywords: CO-releasing materials; carbon monoxide; metal−organic frameworks; molybdenum; pyrazine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
A hafnium-based metal-organic framework for the entrapment of molybdenum hexacarbonyl and the light-responsive release of the gasotransmitter carbon monoxide.Mater Sci Eng C Mater Biol Appl. 2021 May;124:112053. doi: 10.1016/j.msec.2021.112053. Epub 2021 Mar 25. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33947547
-
An organic-organometallic CO-releasing material comprising 4,4'-bipyridine and molybdenum subcarbonyl building blocks.Dalton Trans. 2024 Jul 30;53(30):12783-12796. doi: 10.1039/d4dt01303d. Dalton Trans. 2024. PMID: 39023244
-
Coordination Modulation Method To Prepare New Metal-Organic Framework-Based CO-Releasing Materials.ACS Appl Mater Interfaces. 2018 Sep 19;10(37):31158-31167. doi: 10.1021/acsami.8b11758. Epub 2018 Sep 7. ACS Appl Mater Interfaces. 2018. PMID: 30152684
-
Recent advances in the development of metal-organic framework-based gas-releasing nanoplatforms for synergistic cancer therapy.Dalton Trans. 2021 Feb 2;50(4):1189-1196. doi: 10.1039/d0dt03767b. Dalton Trans. 2021. PMID: 33438684 Review.
-
Metal-organic frameworks for therapeutic gas delivery.Adv Drug Deliv Rev. 2021 Apr;171:199-214. doi: 10.1016/j.addr.2021.02.005. Epub 2021 Feb 6. Adv Drug Deliv Rev. 2021. PMID: 33561450 Review.
Cited by
-
Understanding CO adsorption in MOFs combining atomic simulations and machine learning.Sci Rep. 2024 Oct 22;14(1):24931. doi: 10.1038/s41598-024-76491-x. Sci Rep. 2024. PMID: 39438709 Free PMC article.
-
Transdermal Hydrogen Sulfide Delivery Enabled by Open-Metal-Site Metal-Organic Frameworks.J Am Chem Soc. 2024 Jul 17;146(28):18927-18937. doi: 10.1021/jacs.4c00674. Epub 2024 Jul 5. J Am Chem Soc. 2024. PMID: 38968420 Free PMC article.
-
Pyrazine-bridged polymetallic copper-iridium clusters.Acta Crystallogr E Crystallogr Commun. 2024 Jul 27;80(Pt 8):890-893. doi: 10.1107/S2056989024007151. eCollection 2024 Aug 1. Acta Crystallogr E Crystallogr Commun. 2024. PMID: 39108787 Free PMC article.
References
-
- Ford P. C. Metal complex strategies for photo-uncaging the small molecule bioregulators nitric oxide and carbon monoxide. Coord. Chem. Rev. 2018, 376, 548–564. 10.1016/j.ccr.2018.07.018. - DOI
-
- Xiang H.-J.; Guo M.; Liu J.-G. Transition-metal nitrosyls for photocontrolled nitric oxide delivery. Eur. J. Inorg. Chem. 2017, 1586–1595. 10.1002/ejic.201601135. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

