Clinical Profiles and Patterns of Kidney Disease Progression in C3 Glomerulopathy
- PMID: 36996481
- PMCID: PMC10278771
- DOI: 10.34067/KID.0000000000000115
Clinical Profiles and Patterns of Kidney Disease Progression in C3 Glomerulopathy
Abstract
Key Points:
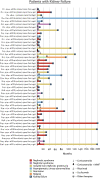
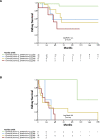
Kidney survival in C3 glomerulopathy is significantly higher in patients with a disease chronicity score <4 and proteinuria <3.5 g/d, regardless of baseline eGFR.
A faster eGFR decline in C3 glomerulopathy is associated with higher probability of kidney failure.
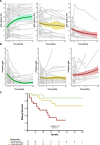
Patients with glomerulopathy with a progressive reduction in proteinuria over time did not reach kidney failure.
Background: C3 glomerulopathy is a rare kidney disease, which makes it difficult to collect large cohorts of patients to better understand its variability. The aims of this study were to describe the clinical profiles and patterns of progression of kidney disease.
Methods: This was a retrospective, observational cohort study. Patients diagnosed with C3 glomerulopathy between 1995 and 2020 were enrolled. Study population was divided into clinical profiles by combining the following predictors: eGFR under/above 30 ml/min per 1.73 m2, proteinuria under/above 3.5 g/d, and histologic chronicity score under/above 4. The change in eGFR and proteinuria over time was evaluated in a subgroup with consecutive measurements of eGFR and proteinuria.
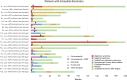
Results: One hundred and fifteen patients with a median age of 30 years (interquartile range 19–50) were included. Patients were divided into eight clinical profiles. Kidney survival was significantly higher in patients with a chronicity score <4 and proteinuria <3.5 g/d, both in those presenting with an eGFR under/above 30 ml/min per 1.73 m2. The median eGFR slope of patients who reached kidney failure was −6.5 ml/min per 1.73 m2 per year (interquartile range −1.6 to −17). Patients who showed a reduction in proteinuria over time did not reach kidney failure. On the basis of the rate of eGFR decline, patients were classified as faster eGFR decline (≥5 ml/min per 1.73 m2 per year), slower (<5 ml/min per 1.73 m2 per year), and those without decline. A faster eGFR decline was associated with higher probability of kidney failure.
Conclusions: Kidney survival is significantly higher in patients with a chronicity score <4 and proteinuria <3.5 g/d regardless of baseline eGFR, and a faster rate of decline in eGFR is associated with higher probability of kidney failure.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

