Clinical efficacy of Azithromycin for COVID-19 management: A systematic meta-analysis of meta-analyses
- PMID: 36996755
- PMCID: PMC10017380
- DOI: 10.1016/j.hrtlng.2023.03.004
Clinical efficacy of Azithromycin for COVID-19 management: A systematic meta-analysis of meta-analyses
Abstract
Background: Azithromycin has been adopted as a component of the COVID-19 management protocol throughout the global healthcare settings but with a questionable if not downright unsubstantiated evidence base.
Objectives: In order to amalgamate and critically appraise the conflicting evidence around the clinical efficacy of Azithromycin (AZO) vis a vis COVID-19 management outcomes, a meta-analysis of meta-analyses was carried out to establish an evidence-based holistic status of AZO vis a vis its efficacy as a component-in-use of the COVID-19 management protocol.
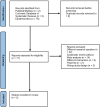
Methods: A comprehensive systematic search was carried out through PubMed/Medline, Cochrane and Epistemonikos with a subsequent appraisal of abstracts and full-texts, as required. The Quality of Reporting of Meta-analyses (QUOROM) checklist and the Assessment of Multiple Systematic Reviews (AMSTAR) methodology were adopted to assess the methodological quality of the included meta-analyses. Random-effects models were developed to calculate summarized pool Odds Ratios (with 95% confidence interval) for the afore determined primary and secondary outcomes.
Results: AZO, when compared with best available therapy (BAT) including or excluding Hydroxychloroquine, exhibited statistically insignificant reduction in mortality [(n= 27,204 patients) OR= 0.77 (95% CI: 0.51-1.16) (I2= 97%)], requirement of mechanical ventilation [(n= 14,908 patients) OR= 1.4 (95% CI: 0.58-3.35) (I2= 98%)], induction of arrhythmia [(n= 9,723 patients) OR= 1.21 (95% CI: 0.63-2.32) (I2= 92%)] and QTc prolongation (a surrogate for torsadogenic effect) [(n= 6,534 patients) OR= 0.62 (95% CI: 0.23-1.73) (I2= 96%)].
Conclusion: The meta-analysis of meta-analyses portrays AZO as a pharmacological agent that does not appear to have a comparatively superior clinical efficacy than BAT when it comes to COVID-19 management. Secondary to a very real threat of anti-bacterial resistance, it is suggested that AZO be discontinued and removed from COVID-19 management protocols.
Keywords: Arrhythmia; Azithromycin; COVID-19; Mortality; Torsades de pointes.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflict of interest.
Figures

References
-
- Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, et al. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021;19:147–163. - PubMed

