Lysine-Derived Charge-Altering Releasable Transporters: Targeted Delivery of mRNA and siRNA to the Lungs
- PMID: 36996808
- PMCID: PMC10601965
- DOI: 10.1021/acs.bioconjchem.3c00019
Lysine-Derived Charge-Altering Releasable Transporters: Targeted Delivery of mRNA and siRNA to the Lungs
Abstract
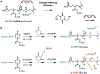
Targeted delivery of nucleic acid therapeutics to the lungs could transform treatment options for pulmonary disease. We have previously developed oligomeric charge-altering releasable transporters (CARTs) for in vivo mRNA transfection and demonstrated their efficacy for use in mRNA-based cancer vaccination and local immunomodulatory therapies against murine tumors. While our previously reported glycine-based CART-mRNA complexes (G-CARTs/mRNA) show selective protein expression in the spleen (mouse, >99%), here, we report a new lysine-derived CART-mRNA complex (K-CART/mRNA) that, without additives or targeting ligands, shows selective protein expression in the lungs (mouse, >90%) following systemic IV administration. We further show that by delivering siRNA using the K-CART, we can significantly decrease expression of a lung-localized reporter protein. Blood chemistry and organ pathology studies demonstrate that K-CARTs are safe and well-tolerated. We report on the new step economical, organocatalytic synthesis (two steps) of functionalized polyesters and oligo-carbonate-co-α-aminoester K-CARTs from simple amino acid and lipid-based monomers. The ability to direct protein expression selectively in the spleen or lungs by simple, modular changes to the CART structure opens fundamentally new opportunities in research and gene therapy.
Conflict of interest statement
The authors declare the following competing financial interest(s): Ronald Levy serves on the Scientific Advisory Boards of Quadriga, BeiGene, GigaGen, Teneobio, Nurix, Dragonfly, Apexigen, Viracta, Spotlight, Immunocore, Walking Fish, Kira, Abintus Bio, Khloris, Virsti, BiolineRx. Paul Wender serves on the Science Advisory Boards of BryoLogyx, N1 Life, Synaptogenix, SuperTrans Medical, Vault Pharma, and Cytokinetics. The other authors have no conflicts of interest to declare.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

