Self-assembled innervated vasculature-on-a-chip to study nociception
- PMID: 36996841
- PMCID: PMC10152403
- DOI: 10.1088/1758-5090/acc904
Self-assembled innervated vasculature-on-a-chip to study nociception
Abstract
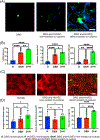
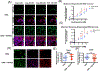
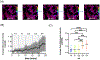
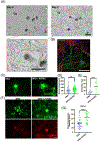
Nociceptor sensory neurons play a key role in eliciting pain. An active crosstalk between nociceptor neurons and the vascular system at the molecular and cellular level is required to sense and respond to noxious stimuli. Besides nociception, interaction between nociceptor neurons and vasculature also contributes to neurogenesis and angiogenesis.In vitromodels of innervated vasculature can greatly help delineate these roles while facilitating disease modeling and drug screening. Herein, we report the development of a microfluidic-assisted tissue model of nociception in the presence of microvasculature. The self-assembled innervated microvasculature was engineered using endothelial cells and primary dorsal root ganglion (DRG) neurons. The sensory neurons and the endothelial cells displayed distinct morphologies in presence of each other. The neurons exhibited an elevated response to capsaicin in the presence of vasculature. Concomitantly, increased transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor expression was observed in the DRG neurons in presence of vascularization. Finally, we demonstrated the applicability of this platform for modeling nociception associated with tissue acidosis. While not demonstrated here, this platform could also serve as a tool to study pain resulting from vascular disorders while also paving the way towards the development of innervated microphysiological models.
Keywords: innervation; organ-on-a-chip; tissue engineering; vascular tissue engineering.
© 2023 IOP Publishing Ltd.
Figures

References
-
- Bhatia SN and Ingber DE 2014. Microfluidic organs-on-chips Nature Biotechnology 32 760 - PubMed
-
- Park D, Lee J, Chung JJ, Jung Y and Kim SH 2020. Integrating Organs-on-Chips: Multiplexing, Scaling, Vascularization, and Innervation Trends Biotechnol 38 99–112 - PubMed
-
- Adriani G, Ma D, Pavesi A, Kamm RD and Goh ELK 2017. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier Lab Chip 17 448–59 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
