Incidence of HIV and hepatitis C virus among people who inject drugs, and associations with age and sex or gender: a global systematic review and meta-analysis
- PMID: 36996853
- PMCID: PMC10817215
- DOI: 10.1016/S2468-1253(23)00018-3
Incidence of HIV and hepatitis C virus among people who inject drugs, and associations with age and sex or gender: a global systematic review and meta-analysis
Abstract
Background: Measuring the incidence of HIV and hepatitis C virus (HCV) infection among people who inject drugs (PWID) is key to track progress towards elimination. We aimed to summarise global data on HIV and primary HCV incidence among PWID and associations with age and sex or gender.
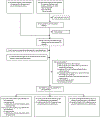
Methods: In this systematic review and meta-analysis, we updated an existing database of HIV and HCV incidence studies among PWID by searching MEDLINE, Embase, and PsycINFO, capturing studies published between Jan 1, 2000, and Dec 12, 2022, with no language or study design restrictions. We contacted authors of identified studies for unpublished or updated data. We included studies that estimated incidence by longitudinally re-testing people at risk of infection or by using assays for recent infection. We pooled incidence and relative risk (RR; young [generally defined as ≤25 years] vs older PWID; women vs men) estimates using random-effects meta-analysis and assessed risk of bias with a modified Newcastle-Ottawa scale. This study is registered with PROSPERO, CRD42020220884.
Findings: Our updated search identified 9493 publications, of which 211 were eligible for full-text review. An additional 377 full-text records from our existing database and five records identified through cross-referencing were assessed. Including 28 unpublished records, 125 records met the inclusion criteria. We identified 64 estimates of HIV incidence (30 from high-income countries [HICs] and 34 from low-income or middle-income countries [LMICs]) and 66 estimates of HCV incidence (52 from HICs and 14 from LMICs). 41 (64%) of 64 HIV and 42 (64%) of 66 HCV estimates were from single cities rather than being multi-city or nationwide. Estimates were measured over 1987-2021 for HIV and 1992-2021 for HCV. Pooled HIV incidence was 1·7 per 100 person-years (95% CI 1·3-2·3; I2=98·4%) and pooled HCV incidence was 12·1 per 100 person-years (10·0-14·6; I2=97·2%). Young PWID had a greater risk of HIV (RR 1·5, 95% CI 1·2-1·8; I2=66·9%) and HCV (1·5, 1·3-1·8; I2=70·6%) acquisition than older PWID. Women had a greater risk of HIV (RR 1·4, 95% CI 1·1-1·6; I2=55·3%) and HCV (1·2, 1·1-1·3; I2=43·3%) acquisition than men. For both HIV and HCV, the median risk-of-bias score was 6 (IQR 6-7), indicating moderate risk.
Interpretation: Although sparse, available HIV and HCV incidence estimates offer insights into global levels of HIV and HCV transmission among PWID. Intensified efforts are needed to keep track of the HIV and HCV epidemics among PWID and to expand access to age-appropriate and gender-appropriate prevention services that serve young PWID and women who inject drugs.
Funding: Canadian Institutes of Health Research, Fonds de recherche du Québec-Santé, Canadian Network on Hepatitis C, UK National Institute for Health and Care Research, and WHO.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AA acknowledges support through postdoctoral fellowships from the Canadian Institute of Health Research (CIHR), Fonds de recherche du Québec – Santé (FRQ-S), and the Canadian Network on Hepatitis C. MA reports grants paid to his institution from the Public Health Agency of Canada and grants from the Ministère de la santé et des services sociaux du Québec, during the conduct of this study, and grants from the CIHR, outside the submitted work. JA reports grants from the National Institutes of Health (NIH), during the conduct of this study. JB reports consulting fees from Gilead Sciences, AbbVie, and Cepheid; payment or honoraria from Gilead Sciences for an educational event; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Gilead Sciences, as part of an NIH R01 grant, outside of the submitted work. CSC reports grants from Gilead Sciences, Janssen Pharmaceuticals, and GSK (paid to the University of Calgary); consulting fees from Roche Pharmaceuticals, Gilead Sciences, Janssen Pharmaceuticals (paid to the University of Calgary, on behalf of the Canadian Hepatitis B Virus [HBV] Network), and Altimmune (paid to the University of Calgary, on behalf of the Canadian HBV Network); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events through Gilead Sciences; and patents planned, issued, or pending (PCT/CA2021/050234; international patent application title: polypeptides directed against viral infection and uses thereof; compositions and methodologies for the detection and treatment of HBV infection; USA; 62/982,474; Feb 28, 2020). SC reports payments for chairing or presenting at a European HIV Testing Week webinar from the Centre of Excellence for Health, Immunity and Infections, outside the submitted work. KH reports financial support for the present manuscript paid to her institution from the US National Institute of Drug Abuse (NIDA; Vancouver Drug Users Study: the impacts of evolving drug use patterns on HIV/AIDS; U01DA038886), the Michael Smith Foundation for Health Research Scholar Award, and St Paul's Foundation, and grants or contracts from the CIHR and the William and Ada Isabelle Steel Fund through Simon Fraser University (paid to institution) and the British Columbia Centre on Substance Use (paid to KH). GM reports grants or contracts from AbbVie (clinic support, advisory board, and speaker fees), the Canadian Network on Hepatitis C (research support), Coverdale (clinic support), Gilead Sciences (clinic support and speakers fees), International Network on Health and Hepatitis in Substance Users (INHSU; speaker fees), and TD Bank (clinic support); payment or honoraria from the Canadian Association for the Study of the Liver (scientific planning committee for web-based learning, conjoint with AbbVie; payment to institution), AbbVie (speakers fees for educational podcasts and brochure; payment partly to institution and partly to GM), Gilead Sciences (speaker fees; payment partly to institution and partly to GM), and ECHO plus (honorarium for speaking); and support for attending meetings or travel, or both, from INHSU and being on the advisory board for AbbVie and Gilead. JGW reports grants and contracts from Gilead Sciences and FIND, The Global Alliance for Diagnostics. MH reports having a leadership or fiduciary role as trustee of the Society for the Study of Addiction. All other authors declare no competing interests. Declaration of interests for the members of the HIV and HCV Incidence Review Collaborative Group are provided in the appendix (p 52).
Figures






Comment in
-
The road to HIV and HCV elimination among people who inject drugs.Lancet Gastroenterol Hepatol. 2023 Jun;8(6):497-498. doi: 10.1016/S2468-1253(23)00082-1. Epub 2023 Mar 27. Lancet Gastroenterol Hepatol. 2023. PMID: 36996851 No abstract available.
References
-
- UNAIDS. 90–90–90 An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS, 2014. https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf(accessed May 5, 2020).
-
- WHO. Global health sector strategy on viral hepatitis 2016–2021. Geneva, Switzerland: World Health Organization, 2016. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pd... (accessed Aug 5, 2017).
Publication types
MeSH terms
Grants and funding
- R01 DA047952/DA/NIDA NIH HHS/United States
- L60 DA056996/DA/NIDA NIH HHS/United States
- DH_/Department of Health/United Kingdom
- R01 AI147490/AI/NIAID NIH HHS/United States
- DP2 DA056130/DA/NIDA NIH HHS/United States
- R21 DA046809/DA/NIDA NIH HHS/United States
- 001/WHO_/World Health Organization/International
- R01 DA033679/DA/NIDA NIH HHS/United States
- MC_UU_00004/03/MRC_/Medical Research Council/United Kingdom
- U01 DA038886/DA/NIDA NIH HHS/United States
- MR/N00616X/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- R01 DA037773/DA/NIDA NIH HHS/United States
- R01 DA041736/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

