Live and pasteurized Akkermansia muciniphila decrease susceptibility to Salmonella Typhimurium infection in mice
- PMID: 36996967
- PMCID: PMC10555781
- DOI: 10.1016/j.jare.2023.03.008
Live and pasteurized Akkermansia muciniphila decrease susceptibility to Salmonella Typhimurium infection in mice
Abstract
Introduction: The gut microbiome is vital for providing resistance against colonized pathogenicbacteria. Recently, specific commensal species have become recognized as important mediators of host defense against microbial infection by a variety of mechanisms.
Objectives: To examine the contribution of live and pasteurized A. muciniphila to defend against the intestinal pathogen Salmonella Typhimurium in a streptomycin-treated mouse model of infection.
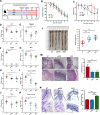
Methods: C57B6J mice were pretreated with phosphate-buffered saline (PBS), live Akkermansia muciniphila (AKK), and pasteurized A. muciniphila (pAKK) for two weeks, then mice were infected by S. Typhimurium SL 1344. 16S rRNA-based gut microbiota analysis was performed before and after infection. Bacterial counts in feces and tissues, histopathological analysis, gut barrier-related gene expression, and antimicrobial peptides were examined. Co-housing was performed to examine the role of microbiota in the change of susceptibility of mice to infection.
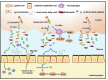
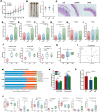
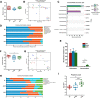
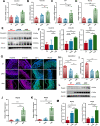
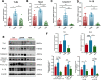
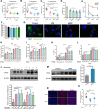
Results: AKK and pAKK markedly decreased Salmonella fecal and systemic burdens and reduced inflammation during infection. Notably, further characterization of AKK and pAKK protective mechanisms revealed different candidate protective pathways. AKK promoted gutbarrier gene expression and the secretion of antimicrobial peptides, and co-housing studies suggested that AKK-associated microbial community played a role in attenuating infection. Moreover, pAKK had a positive effect on NLRP3 in infected mice. We verified that pretreatment of pAKK could promote the expression of NLRP3, and enhance the antimicrobial activity of macrophage, likely through increasing the production of reactive oxygen (ROS), nitric oxide (NO), and inflammatory cytokines.
Conclusion: Our study demonstrates that live or pasteurized A. muciniphila can be effective preventive measures for alleviating S. Typhimurium-induced disease, highlighting the potential of developing Akkermansia-based probiotics or postbiotics for the prevention of Salmonellosis.
Keywords: Akkermansia muciniphila; Gut barriers; Gut microbiota; Inflammasomes; RegIII lectins; Salmonella Typhimurium.
Copyright © 2023. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Pasteurized Akkermansia muciniphila Ameliorates Preeclampsia in Mice by Enhancing Gut Barrier Integrity, Improving Endothelial Function, and Modulating Gut Metabolic Dysregulation.Microorganisms. 2024 Dec 2;12(12):2483. doi: 10.3390/microorganisms12122483. Microorganisms. 2024. PMID: 39770686 Free PMC article.
-
Pasteurized Akkermansia muciniphila ameliorates preeclampsia via inhibiting mitochondrial dysfunction-mediated placental apoptosis in vivo and in vitro.Free Radic Biol Med. 2025 Jul;234:233-247. doi: 10.1016/j.freeradbiomed.2025.04.044. Epub 2025 Apr 26. Free Radic Biol Med. 2025. PMID: 40294855
-
Pasteurized Akkermansia muciniphila protects from fat mass gain but not from bone loss.Am J Physiol Endocrinol Metab. 2020 Apr 1;318(4):E480-E491. doi: 10.1152/ajpendo.00425.2019. Epub 2020 Jan 21. Am J Physiol Endocrinol Metab. 2020. PMID: 31961709 Free PMC article.
-
[Advances of Akkermansia muciniphila in regulating host functions].Zhongguo Zhong Yao Za Zhi. 2021 Jun;46(11):2760-2765. doi: 10.19540/j.cnki.cjcmm.20210119.601. Zhongguo Zhong Yao Za Zhi. 2021. PMID: 34296573 Review. Chinese.
-
Akkermansia muciniphila plays critical roles in host health.Crit Rev Microbiol. 2023 Feb;49(1):82-100. doi: 10.1080/1040841X.2022.2037506. Epub 2022 May 21. Crit Rev Microbiol. 2023. PMID: 35603929 Review.
Cited by
-
Lacticaseibacillus rhamnosus P118 enhances host tolerance to Salmonella infection by promoting microbe-derived indole metabolites.Elife. 2025 Aug 7;13:RP101198. doi: 10.7554/eLife.101198. Elife. 2025. PMID: 40773367 Free PMC article.
-
Gut Microbiota at the Crossroad of Hepatic Oxidative Stress and MASLD.Antioxidants (Basel). 2025 Jan 6;14(1):56. doi: 10.3390/antiox14010056. Antioxidants (Basel). 2025. PMID: 39857390 Free PMC article. Review.
-
Akkermansia muciniphila Alleviates Porphyromonas gingivalis-induced Periodontal Disease by Enhancing Bacterial Clearance.Probiotics Antimicrob Proteins. 2025 Apr 29. doi: 10.1007/s12602-025-10541-2. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40299200
-
Therapeutic Approach Targeting Gut Microbiome in Gastrointestinal Infectious Diseases.Int J Mol Sci. 2023 Oct 27;24(21):15654. doi: 10.3390/ijms242115654. Int J Mol Sci. 2023. PMID: 37958637 Free PMC article. Review.
-
Co-housing with Tibetan chickens improved the resistance of Arbor Acres chickens to Salmonella enterica serovar Enteritidis infection by altering their gut microbiota composition.J Anim Sci Biotechnol. 2025 Jan 3;16(1):2. doi: 10.1186/s40104-024-01132-2. J Anim Sci Biotechnol. 2025. PMID: 39748400 Free PMC article.
References
-
- Vieira K., Silva H., Rocha I., Barboza E., Eller L. Foodborne pathogens in the omics era. Crit Rev Food Sci Nutr. 2021;1–16 - PubMed
-
- Sellin M.E., Müller A.A., Felmy B., Dolowschiak T., Diard M., Tardivel A., et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe. 2014;16(2):237–248. - PubMed
-
- Muruve D.A., Pétrilli V., Zaiss A.K., White L.R., Clark S.A., Ross P.J., et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452(7183):103–107. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

