A Spontaneous Melanoma Mouse Model Applicable for a Longitudinal Chemotherapy and Immunotherapy Study
- PMID: 36997110
- PMCID: PMC10524215
- DOI: 10.1016/j.jid.2023.03.1664
A Spontaneous Melanoma Mouse Model Applicable for a Longitudinal Chemotherapy and Immunotherapy Study
Abstract
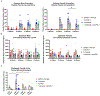
Mouse models that reflect human disorders provide invaluable tools for the translation of basic science discoveries to clinical therapies. However, many of these in vivo therapeutic studies are short term and do not accurately mimic patient conditions. In this study, we used a fully immunocompetent, transgenic mouse model, TGS, in which the spontaneous development of metastatic melanoma is driven by the ectopic expression of a normal neuronal receptor, mGluR1, as a model to assess longitudinal treatment response (up to 8 months) with an inhibitor of glutamatergic signaling, troriluzole, which is a prodrug of riluzole, plus an antibody against PD-1, an immune checkpoint inhibitor. Our results reveal a sex-biased treatment response that led to improved survival in troriluzole and/or anti-PD-1-treated male mice that correlated with differential CD8+ T cells and CD11b+ myeloid cell populations in the tumor-stromal interface, supporting the notion that this model is a responsive and tractable system for evaluating therapeutic regimens for melanoma in an immunocompetent setting.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- André T, Cohen R, Salem ME. Immune Checkpoint Blockade Therapy in Patients With Colorectal Cancer Harboring Microsatellite Instability/Mismatch Repair Deficiency in 2022. Am Soc Clin Oncol Educ Book 2022;42:1–9. - PubMed
-
- André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med 2020;383(23):2207–18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

