The C-terminal tail of polycystin-1 suppresses cystic disease in a mitochondrial enzyme-dependent fashion
- PMID: 36997516
- PMCID: PMC10063565
- DOI: 10.1038/s41467-023-37449-1
The C-terminal tail of polycystin-1 suppresses cystic disease in a mitochondrial enzyme-dependent fashion
Abstract
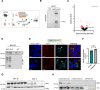
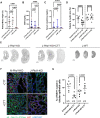
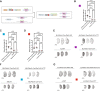
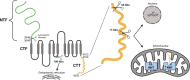
Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent potentially lethal monogenic disorder. Mutations in the PKD1 gene, which encodes polycystin-1 (PC1), account for approximately 78% of cases. PC1 is a large 462-kDa protein that undergoes cleavage in its N and C-terminal domains. C-terminal cleavage produces fragments that translocate to mitochondria. We show that transgenic expression of a protein corresponding to the final 200 amino acid (aa) residues of PC1 in two Pkd1-KO orthologous murine models of ADPKD suppresses cystic phenotype and preserves renal function. This suppression depends upon an interaction between the C-terminal tail of PC1 and the mitochondrial enzyme Nicotinamide Nucleotide Transhydrogenase (NNT). This interaction modulates tubular/cyst cell proliferation, the metabolic profile, mitochondrial function, and the redox state. Together, these results suggest that a short fragment of PC1 is sufficient to suppress cystic phenotype and open the door to the exploration of gene therapy strategies for ADPKD.
© 2023. The Author(s).
Conflict of interest statement
Some of the findings presented in this manuscript are included in the provisional patent application No 63/250,663 filed by Yale University that includes L.O., V.P. and M.J.C. as authors. The remaining authors declare no other competing interests.
Figures




References
-
- The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. The European Polycystic Kidney Disease Consortium. Cell78, 725 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

