Preclinical development of a first-in-class vaccine encoding HER2, Brachyury and CD40L for antibody enhanced tumor eradication
- PMID: 36997583
- PMCID: PMC10060934
- DOI: 10.1038/s41598-023-32060-2
Preclinical development of a first-in-class vaccine encoding HER2, Brachyury and CD40L for antibody enhanced tumor eradication
Abstract
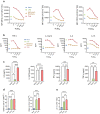
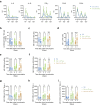
The induction of antiviral innate immunity by systemic immunization with live virus can be employed to positively impact the response to therapeutic vaccination. We previously demonstrated that systemic immunization with a non-replicating MVA encoding CD40 ligand (CD40L) enhances innate immune cell activation and function, and triggers potent antitumor CD8+ T cell responses in different murine tumor models. Antitumor efficacy was increased when combined with tumor targeting antibodies. Here we report the development of TAEK-VAC-HerBy (TVH), a first-in-class human tumor antibody enhanced killing (TAEK) vaccine based on the non-replicating MVA-BN viral vector. It encodes the membrane bound form of human CD40L, HER2 and the transcription factor Brachyury. TVH is designed for therapeutic use in HER2- or Brachyury-expressing cancer patients in combination with tumor targeting antibodies. To preclude possible oncogenic activities in infected cells and to prevent binding of vaccine-encoded HER2 by monoclonal antibodies trastuzumab and pertuzumab, genetic modifications of HER2 were introduced in the vaccine. Brachyury was genetically modified to prevent nuclear localization of the protein thereby inhibiting its transcriptional activity. CD40L encoded in TVH enhanced human leukocyte activation and cytokine secretion in vitro. Lastly, TVH intravenous administration to non-human primates was proven immunogenic and safe in a repeat-dose toxicity study. Nonclinical data presented here highlight TVH as a first-in-class immunotherapeutic vaccine platform currently under clinical investigation.
© 2023. The Author(s).
Conflict of interest statement
The authors enlisted are or have been employees of Bavarian Nordic.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

