G-CSF drives autoinflammation in APLAID
- PMID: 36997670
- PMCID: PMC10154231
- DOI: 10.1038/s41590-023-01473-6
G-CSF drives autoinflammation in APLAID
Abstract
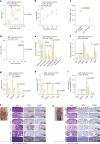
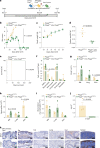
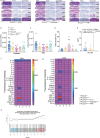
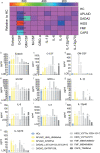
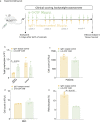
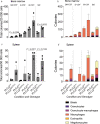
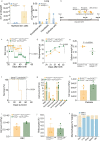
Missense mutations in PLCG2 can cause autoinflammation with phospholipase C gamma 2-associated antibody deficiency and immune dysregulation (APLAID). Here, we generated a mouse model carrying an APLAID mutation (p.Ser707Tyr) and found that inflammatory infiltrates in the skin and lungs were only partially ameliorated by removing inflammasome function via the deletion of caspase-1. Also, deleting interleukin-6 or tumor necrosis factor did not fully prevent APLAID mutant mice from autoinflammation. Overall, these findings are in accordance with the poor response individuals with APLAID have to treatments that block interleukin-1, JAK1/2 or tumor necrosis factor. Cytokine analysis revealed increased granulocyte colony-stimulating factor (G-CSF) levels as the most distinct feature in mice and individuals with APLAID. Remarkably, treatment with a G-CSF antibody completely reversed established disease in APLAID mice. Furthermore, excessive myelopoiesis was normalized and lymphocyte numbers rebounded. APLAID mice were also fully rescued by bone marrow transplantation from healthy donors, associated with reduced G-CSF production, predominantly from non-hematopoietic cells. In summary, we identify APLAID as a G-CSF-driven autoinflammatory disease, for which targeted therapy is feasible.
© 2023. The Author(s).
Conflict of interest statement
S.L.M. is a Scientific Advisor for Odyssey Therapeutics and NRG Therapeutics. S.-L.N. and J.N. are employees of GSK. I.P.W. has acted as a Scientific Advisor for CSL and received funding for research on G-CSF antagonism in inflammatory diseases. All other authors declare no competing interests.
Figures








Comment in
-
Targeting G-CSF to treat autoinflammation.Nat Immunol. 2023 May;24(5):736-737. doi: 10.1038/s41590-023-01474-5. Nat Immunol. 2023. PMID: 36997672 No abstract available.
-
G-CSF implicated as therapeutic target in APLAID.Nat Rev Rheumatol. 2023 Jun;19(6):326. doi: 10.1038/s41584-023-00973-x. Nat Rev Rheumatol. 2023. PMID: 37161085 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

