Tumor Immunophenotyping-Derived Signature Identifies Prognosis and Neoadjuvant Immunotherapeutic Responsiveness in Gastric Cancer
- PMID: 36998102
- PMCID: PMC10214263
- DOI: 10.1002/advs.202207417
Tumor Immunophenotyping-Derived Signature Identifies Prognosis and Neoadjuvant Immunotherapeutic Responsiveness in Gastric Cancer
Abstract
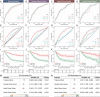
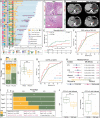
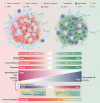
The effectiveness of neoadjuvant immune checkpoint inhibitor (ICI) therapy is confirmed in clinical trials; however, the patients suitable for receiving this therapy remain unspecified. Previous studies have demonstrated that the tumor microenvironment (TME) dominates immunotherapy; therefore, an effective TME classification strategy is required. In this study, five crucial immunophenotype-related molecules (WARS, UBE2L6, GZMB, BATF2, and LAG-3) in the TME are determined in five public gastric cancer (GC) datasets (n = 1426) and an in-house sequencing dataset (n = 79). Based on this, a GC immunophenotypic score (IPS) is constructed using the least absolute shrinkage and selection operator (LASSO) Cox, and randomSurvivalForest. IPSLow is characterized as immune-activated, and IPSHigh is immune-silenced. Data from seven centers (n = 1144) indicate that the IPS is a robust and independent biomarker for GC and superior to the AJCC stage. Furthermore, patients with an IPSLow and a combined positive score of ≥5 are likely to benefit from neoadjuvant anti-PD-1 therapy. In summary, the IPS can be a useful quantitative tool for immunophenotyping to improve clinical outcomes and provide a practical reference for implementing neoadjuvant ICI therapy for patients with GC.
Keywords: gastric cancer; immune contexture; neoadjuvant immune checkpoint inhibitor therapy; prognosis; tumor microenvironment.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Petricevic B., Kabiljo J., Zirnbauer R., Walczak H., Laengle J., Bergmann M., Semin. Cancer Biol. 2022, 86, 834. - PubMed
-
- Lee K. W., Van Cutsem E., Bang Y. J., Fuchs C. S., Kudaba I., Garrido M., Chung H. C., Lee J., Castro H. R., Chao J., Wainberg Z. A., Cao Z. A., Aurora‐Garg D., Kobie J., Cristescu R., Bhagia P., Shah S., Tabernero J., Shitara K., Wyrwicz L., Clin. Cancer Res. 2022, 28, 3489. - PubMed
-
- Kang Y., Chen L., Ryu M., Oh D., Oh S., Chung H., Lee K., Omori T., Shitara K., Sakuramoto S., Chung I., Yamaguchi K., Kato K., Sym S., Kadowaki S., Tsuji K., Chen J., Bai L., Oh S., Choda Y., Yasui H., Takeuchi K., Hirashima Y., Hagihara S., Boku N., Lancet Oncol. 2022, 23, 234. - PubMed
-
- a) Shitara K., Ajani J. A., Moehler M., Garrido M., Gallardo C., Shen L., Yamaguchi K., Wyrwicz L., Skoczylas T., Bragagnoli A. C., Liu T., Tehfe M., Elimova E., Bruges R., Zander T., de Azevedo S., Kowalyszyn R., Pazo‐Cid R., Schenker M., Cleary J. M., Yanez P., Feeney K., Karamouzis M. V., Poulart V., Lei M., Xiao H., Kondo K., Li M., Janjigian Y. Y., Nature 2022, 603, 942; - PMC - PubMed
- b) Xu J., Jiang H., Pan Y., Gu K., Cang S., Han L., Shu Y., Li J., Zhao J., Pan H., Luo S., Qin Y., Guo Q., Bai Y., Ling Y., Guo Y., Li Z., Liu Y., Wang Y., Zhou H., Ann. Oncol. 2021, 32, S1331.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
