Effect of sleep restriction on insulin sensitivity and energy metabolism in postmenopausal women: A randomized crossover trial
- PMID: 36998155
- PMCID: PMC10191900
- DOI: 10.1002/oby.23739
Effect of sleep restriction on insulin sensitivity and energy metabolism in postmenopausal women: A randomized crossover trial
Abstract
Objective: The aim of this study was to investigate the effect of sleep restriction (SR) on insulin sensitivity and energy metabolism in postmenopausal women.
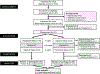
Methods: In a randomized crossover trial, 14 women underwent four nights of habitual sleep (HS, 100% normal sleep) and SR (60% of HS) while following a eucaloric diet. Outcomes included the following: (1) insulin sensitivity by hyperinsulinemic-euglycemic clamp, defined as the glucose infusion rate (GIR); (2) resting metabolism and substrate oxidation by indirect calorimetry; and (3) glucose, insulin, and C-peptide concentrations following a standard meal test.
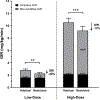
Results: Nine postmenopausal women (mean [SD], age 59 [4] years, BMI 28.0 [2.6] kg/m2 ) were analyzed. Accelerometer-determined total time in bed was 8.4 ± 0.6 hours during HS versus 5.0 ± 0.4 hours during SR (38% reduction, p < 0.0001). SR reduced low-dose insulin GIR by 20% (HS: 2.55 ± 0.22 vs. SR: 2.03 ± 0.20 mg/kg/min; p = 0.01) and high-dose insulin GIR by 12% (HS: 10.48 ± 0.72 vs. SR: 9.19 ± 0.72 mg/kg/min; p < 0.001). SR reduced fat oxidation during high-dose insulin infusion (p < 0.01), and it did not alter resting energy metabolism.
Conclusions: Four nights of SR reduced insulin sensitivity and fat oxidation in postmenopausal women. These findings underscore the role of insufficient sleep in metabolic dysfunction following menopause. Larger trials investigating how sleep disturbances cause metabolic dysfunction during menopause are needed across all stages of menopause.
Trial registration: ClinicalTrials.gov NCT04286451.
© 2023 The Obesity Society.
Conflict of interest statement
Figures
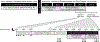
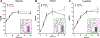
References
-
- El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020;142: e506–e532. - PubMed
-
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354: 1435–1439. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

