Trends in industrialization of biotherapeutics: a survey of product characteristics of 89 antibody-based biotherapeutics
- PMID: 36998195
- PMCID: PMC10072077
- DOI: 10.1080/19420862.2023.2191301
Trends in industrialization of biotherapeutics: a survey of product characteristics of 89 antibody-based biotherapeutics
Abstract
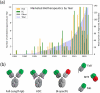
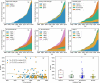
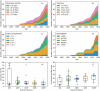
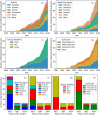
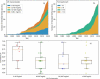
There is considerable interest in the pharmaceutical industry toward development of antibody-based biotherapeutics because they can selectively bind diverse receptors and often possess desirable pharmacology. Here, we studied product characteristics of 89 marketed antibody-based biotherapeutics that were approved from 1986 to mid-2020 by gathering publicly available information. Our analyses revealed major trends in their emergence as the best-selling class of pharmaceuticals. Early on, most therapeutic monoclonal antibodies were developed to treat cancer, with CD20 being the most common target. Thanks to industrialization of antibody manufacturing technologies, their use has now blossomed to include 15 different therapeutic areas and nearly 60 targets, and the field is still growing! Drug manufacturers are solidifying their choices regarding types of antibodies and their molecular formats. IgG1 kappa continues to be the most common molecular format among marketed antibody-based biotherapeutics. Most antibody-based biotherapeutics approved since 2015 are either humanized or fully human, but the data we collected do not show a direct correlation between humanness and reported incidence of anti-drug antibodies. Furthermore, there have also been improvements in terms of drug product stability and high concentration liquid formulations suitable for subcutaneous route of administration, which are being approved more often in recent years. These improvements, however, have not been uniformly adopted across all therapeutic areas, suggesting that multiple options for drug product development are being used to serve diverse therapeutic purposes. Insights gained from this analysis may help us devise better end-to-end antibody-based biotherapeutic drug discovery and development strategies.
Keywords: Antibody; biotherapeutics; developability; drug; formulation; pharmacology.
Conflict of interest statement
All authors were employees of Boehringer-Ingelheim when this research was performed.
Figures

References
-
- Alt N, Zhang TY, Motchnik P, Taticek R, Quarmby V, Schlothauer T, Beck H, Emrich T, Harris RJ.. Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biolog. 2016;44(5):291–305. doi:10.1016/j.biologicals.2016.06.005. PMID: 27461239. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
