Efficacy and Safety of Advanced Therapies for Moderately to Severely Active Ulcerative Colitis at Induction and Maintenance: An Indirect Treatment Comparison Using Bayesian Network Meta-analysis
- PMID: 36998249
- PMCID: PMC10045885
- DOI: 10.1093/crocol/otad009
Efficacy and Safety of Advanced Therapies for Moderately to Severely Active Ulcerative Colitis at Induction and Maintenance: An Indirect Treatment Comparison Using Bayesian Network Meta-analysis
Abstract
Background: Given rapid innovation in advanced therapies for moderately to severely active ulcerative colitis (UC), we investigated their comparative efficacy and safety during induction and maintenance through network meta-analysis.
Methods: Using Bayesian methods, endpoints of clinical remission and clinical response per Full Mayo score, and endoscopic improvement were assessed in bio-naive and -exposed populations. Safety was assessed in overall populations by all adverse events (AEs), serious AEs, discontinuation due to AEs, and serious infections. Phase 3 randomized controlled trials were identified via systematic literature review, including the following advanced therapies: infliximab, adalimumab, vedolizumab, golimumab, tofacitinib, ustekinumab, filgotinib, ozanimod, and upadacitinib. Random effects models were used to address between-study heterogeneity. Intent-to-treat (ITT) efficacy rates were calculated by adjusting maintenance outcomes by likelihood of induction response.
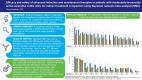
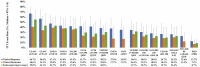
Results: Out of 48 trials identified, 23 were included. Across all outcomes and regardless of prior biologic exposure, ITT efficacy rates were highest for upadacitinib, owing to its highest ranking for all efficacy outcomes in induction and for all but clinical remission during maintenance among bio-naive induction responders. For all advanced therapies versus placebo, there were no significant differences in serious AEs or serious infections across therapies. For all AEs, golimumab had higher odds versus placebo during maintenance; for discontinuation due to AEs, upadacitinib had lower odds versus placebo during induction, while ustekinumab and vedolizumab had lower odds versus placebo during maintenance.
Conclusions: Upadacitinib may be the most efficacious therapy for moderately to severely active UC based on ITT analyses, with similar safety across advanced therapies.
Keywords: advanced therapies; clinical trials; network meta-analysis; ulcerative colitis.
© The Author(s) 2023. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
R.P. has received consulting fees, speaker fees, and research support from AbbVie, Abbott, Alimentiv (formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan Pandion, Pharma, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Schering-Plough, Shire, Sublimity Therapeutics, Theravance Biopharma, UCB, and Takeda Pharmaceuticals. G.Y.M. has consulted for AbbVie, Arena, Boehringer-Ingelheim, Bristol-Meyers Squibb/Celgene, Entasis, Ferring, Janssen, Medtronic, Pfizer, Shionogi, Takeda, Techlab, and has received research grants from Pfizer. S.V. has received research grants from AbbVie, J&J, Pfizer, Galapagos, Takeda and has received consulting and/or speaking fees from AbbVie, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillots Pharma AG, and Zealand Pharma. S.D. has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. P.D.R.H. has received consultancy support from AbbVie, Pfizer, and Eli Lilly; and research grants from AbbVie, Pfizer, and Eli Lilly. Y.S.G., D.I., D.S., and W.Z. are AbbVie employees and may own AbbVie stock and/or options. E.B.C., C.S.K., and S.-T.W. have received consulting fees and research support from AbbVie.
Figures



References
-
- Lynch WD, Hsu R.. Ulcerative colitis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. - PubMed
-
- US Food and Drug Administration. Pfizer Laboratories Div Pfizer Inc. Xeljanz (tofacitinib) [package insert]. Updated December 17, 2021. Accessed January 11, 2021. https://www.accessdata.fda.gov/spl/data/8462e021-ee43-4555-b62b-e8d73920...
-
- US Food and Drug Administration. AbbVie Inc. RINVOQ® (upadacitinib) [package insert]. Updated March 16, 2022. Accessed March 22, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s007lbl.pdf
LinkOut - more resources
Full Text Sources
