In vivo active-targeting fluorescence molecular imaging with adaptive background fluorescence subtraction
- PMID: 36998445
- PMCID: PMC10043309
- DOI: 10.3389/fonc.2023.1130155
In vivo active-targeting fluorescence molecular imaging with adaptive background fluorescence subtraction
Abstract
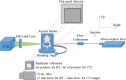
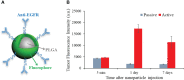
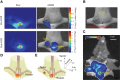
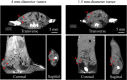
Using active tumor-targeting nanoparticles, fluorescence imaging can provide highly sensitive and specific tumor detection, and precisely guide radiation in translational radiotherapy study. However, the inevitable presence of non-specific nanoparticle uptake throughout the body can result in high levels of heterogeneous background fluorescence, which limits the detection sensitivity of fluorescence imaging and further complicates the early detection of small cancers. In this study, background fluorescence emanating from the baseline fluorophores was estimated from the distribution of excitation light transmitting through tissues, by using linear mean square error estimation. An adaptive masked-based background subtraction strategy was then implemented to selectively refine the background fluorescence subtraction. First, an in vivo experiment was performed on a mouse intratumorally injected with passively targeted fluorescent nanoparticles, to validate the reliability and robustness of the proposed method in a stringent situation wherein the target fluorescence was overlapped with the strong background. Then, we conducted in vivo studies on 10 mice which were inoculated with orthotopic breast tumors and intravenously injected with actively targeted fluorescent nanoparticles. Results demonstrated that active targeting combined with the proposed background subtraction method synergistically increased the accuracy of fluorescence molecular imaging, affording sensitive tumor detection.
Keywords: active targeting; contrast CT; fluorescence background subtraction; fluorescence molecular imaging; fluorescence molecular tomography; image-guided irradiation; multi-modality imaging; nanoparticle imaging.
Copyright © 2023 Vega, Hara, Schmidt, Abuhaija, Tao, Dogan, Pollack, Ford and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Optical molecular imaging-guided radiation therapy part 2: Integrated x-ray and fluorescence molecular tomography.Med Phys. 2017 Sep;44(9):4795-4803. doi: 10.1002/mp.12414. Epub 2017 Jul 21. Med Phys. 2017. PMID: 28626876
-
Fluorescence background subtraction technique for hybrid fluorescence molecular tomography/x-ray computed tomography imaging of a mouse model of early stage lung cancer.J Biomed Opt. 2013 May;18(5):56006. doi: 10.1117/1.JBO.18.5.056006. J Biomed Opt. 2013. PMID: 23640077
-
Computed tomography-guided time-domain diffuse fluorescence tomography in small animals for localization of cancer biomarkers.J Vis Exp. 2012 Jul 17;(65):e4050. doi: 10.3791/4050. J Vis Exp. 2012. PMID: 22847515 Free PMC article.
-
Development of a Novel Histone Deacetylase-Targeted Near-Infrared Probe for Hepatocellular Carcinoma Imaging and Fluorescence Image-Guided Surgery.Mol Imaging Biol. 2020 Jun;22(3):476-485. doi: 10.1007/s11307-019-01389-4. Mol Imaging Biol. 2020. PMID: 31228075
-
Identification and usage of fluorescent probes as nanoparticle contrast agents in detecting cancer.Curr Pharm Des. 2013;19(25):4622-40. doi: 10.2174/1381612811319250009. Curr Pharm Des. 2013. PMID: 23363442 Review.
References
LinkOut - more resources
Full Text Sources

