Oxytocin receptors in the Magel2 mouse model of autism: Specific region, age, sex and oxytocin treatment effects
- PMID: 36998737
- PMCID: PMC10043208
- DOI: 10.3389/fnins.2023.1026939
Oxytocin receptors in the Magel2 mouse model of autism: Specific region, age, sex and oxytocin treatment effects
Abstract
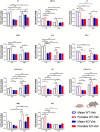
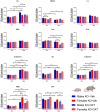
The neurohormone oxytocin (OXT) has been implicated in the regulation of social behavior and is intensively investigated as a potential therapeutic treatment in neurodevelopmental disorders characterized by social deficits. In the Magel2-knockout (KO) mouse, a model of Schaaf-Yang Syndrome, an early postnatal administration of OXT rescued autistic-like behavior and cognition at adulthood, making this model relevant for understanding the actions of OXT in (re)programming postnatal brain development. The oxytocin receptor (OXTR), the main brain target of OXT, was dysregulated in the hippocampus of Magel2-KO adult males, and normalized upon OXT treatment at birth. Here we have analyzed male and female Magel2-KO brains at postnatal day 8 (P8) and at postnatal day 90 (P90), investigating age, genotype and OXT treatment effects on OXTR levels in several regions of the brain. We found that, at P8, male and female Magel2-KOs displayed a widespread, substantial, down-regulation of OXTR levels compared to wild type (WT) animals. Most intriguingly, the postnatal OXT treatment did not affect Magel2-KO OXTR levels at P8 and, consistently, did not rescue the ultrasonic vocalization deficits observed at this age. On the contrary, the postnatal OXT treatment reduced OXTR levels at P90 in male Magel2-KO in a region-specific way, restoring normal OXTR levels in regions where the Magel2-KO OXTR was upregulated (central amygdala, hippocampus and piriform cortex). Interestingly, Magel2-KO females, previously shown to lack the social deficits observed in Magel2-KO males, were characterized by a different trend in receptor expression compared to males; as a result, the dimorphic expression of OXTR observed in WT animals, with higher OXTR expression observed in females, was abolished in Magel2-KO mice. In conclusion, our data indicate that in Magel2-KO mice, OXTRs undergo region-specific modifications related to age, sex and postnatal OXT treatment. These results are instrumental to design precisely-timed OXT-based therapeutic strategies that, by acting at specific brain regions, could modify the outcome of social deficits in Schaaf-Yang Syndrome patients.
Keywords: Prader-Willi Syndrome (PWS); Schaaf-Yang Syndrome; neurodevelopmental disorders (NDD); oxytocin receptor expression; postnatal oxytocin treatment.
Copyright © 2023 Gigliucci, Busnelli, Santini, Paolini, Bertoni, Schaller, Muscatelli and Chini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

