Adenosine deaminase augments SARS-CoV-2 specific cellular and humoral responses in aged mouse models of immunization and challenge
- PMID: 36999023
- PMCID: PMC10043169
- DOI: 10.3389/fimmu.2023.1138609
Adenosine deaminase augments SARS-CoV-2 specific cellular and humoral responses in aged mouse models of immunization and challenge
Abstract
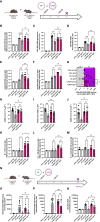
Despite numerous clinically available vaccines and therapeutics, aged patients remain at increased risk for COVID-19 morbidity. Furthermore, various patient populations, including the aged can have suboptimal responses to SARS-CoV-2 vaccine antigens. Here, we characterized vaccine-induced responses to SARS-CoV-2 synthetic DNA vaccine antigens in aged mice. Aged mice exhibited altered cellular responses, including decreased IFNγ secretion and increased TNFα and IL-4 secretion suggestive of TH2-skewed responses. Aged mice exhibited decreased total binding and neutralizing antibodies in their serum but significantly increased TH2-type antigen-specific IgG1 antibody compared to their young counterparts. Strategies to enhance vaccine-induced immune responses are important, especially in aged patient populations. We observed that co-immunization with plasmid-encoded adenosine deaminase (pADA)enhanced immune responses in young animals. Ageing is associated with decreases in ADA function and expression. Here, we report that co-immunization with pADA enhanced IFNγ secretion while decreasing TNFα and IL-4 secretion. pADA expanded the breadth and affinity SARS-CoV-2 spike-specific antibodies while supporting TH1-type humoral responses in aged mice. scRNAseq analysis of aged lymph nodes revealed that pADA co-immunization supported a TH1 gene profile and decreased FoxP3 gene expression. Upon challenge, pADA co-immunization decreased viral loads in aged mice. These data support the use of mice as a model for age-associated decreased vaccine immunogenicity and infection-mediated morbidity and mortality in the context of SARS-CoV-2 vaccines and provide support for the use of adenosine deaminase as a molecular adjuvant in immune-challenged populations.
Keywords: DNA vaccine; SARS; adenosine deaminase (ADA); adjuvant; age.
Copyright © 2023 Gary, Tursi, Warner, Cuismano, Connors, Parzych, Griffin, Bell, Ali, Frase, Hojecki, Canziani, Chaiken, Kannan, Moffat, Embury-Hyatt, Wooton, Kossenkov, Patel, Kobasa, Kutzler, Haddad and Weiner.
Conflict of interest statement
DW has received grant funding, participates in industry collaborations, has received speaking honoraria, and has received fees for consulting, including serving on scientific review committees. Remunerations received by DW include direct payments and equity/options. DW also discloses the following associations with commercial partners: Geneos consultant/advisory board, AstraZeneca advisory board, speaker, Inovio board of directors, consultant, Sanofi advisory board, BBI advisory board, Pfizer advisory Board, Flagship consultant, and Advaccine consultant. SW is a scientific founder of Avamab Pharma Inc., a pre-clinical, pre-revenue stage company dedicated to research and development of AAV gene therapies for the treatment and prevention of infectious diseases. SW is a co-founder and Chief Scientific Officer of Inspire Biotherapeutics, a pre-clinical, pre-revenue stage gene therapy company developing AAV-based therapies for monogenic lung diseases. SW is an inventor on issued patents in Canada and US for the AAV6.2FF capsid, which are owned by the University of Guelph, and licensed to Avamab Pharma Inc., Inspire Biotherapeutics, and Cellastra Inc. From 2020 to February 2023, SW was an unpaid scientific advisor for Cellastra Inc., which is dedicated to research and development of gene therapies targeting root causes of scarring. SW is a co-inventor on a pending US and Canadian patent for the Engineered Newcastle disease virus vector and uses thereof, filed/owned by the University of Guelph. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

