Risk assessment of N- nitrosamines in food
- PMID: 36999063
- PMCID: PMC10043641
- DOI: 10.2903/j.efsa.2023.7884
Risk assessment of N- nitrosamines in food
Abstract
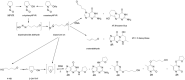
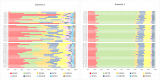
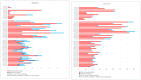
EFSA was asked for a scientific opinion on the risks to public health related to the presence of N-nitrosamines (N-NAs) in food. The risk assessment was confined to those 10 carcinogenic N-NAs occurring in food (TCNAs), i.e. NDMA, NMEA, NDEA, NDPA, NDBA, NMA, NSAR, NMOR, NPIP and NPYR. N-NAs are genotoxic and induce liver tumours in rodents. The in vivo data available to derive potency factors are limited, and therefore, equal potency of TCNAs was assumed. The lower confidence limit of the benchmark dose at 10% (BMDL10) was 10 μg/kg body weight (bw) per day, derived from the incidence of rat liver tumours (benign and malignant) induced by NDEA and used in a margin of exposure (MOE) approach. Analytical results on the occurrence of N-NAs were extracted from the EFSA occurrence database (n = 2,817) and the literature (n = 4,003). Occurrence data were available for five food categories across TCNAs. Dietary exposure was assessed for two scenarios, excluding (scenario 1) and including (scenario 2) cooked unprocessed meat and fish. TCNAs exposure ranged from 0 to 208.9 ng/kg bw per day across surveys, age groups and scenarios. 'Meat and meat products' is the main food category contributing to TCNA exposure. MOEs ranged from 3,337 to 48 at the P95 exposure excluding some infant surveys with P95 exposure equal to zero. Two major uncertainties were (i) the high number of left censored data and (ii) the lack of data on important food categories. The CONTAM Panel concluded that the MOE for TCNAs at the P95 exposure is highly likely (98-100% certain) to be less than 10,000 for all age groups, which raises a health concern.
Keywords: N‐nitrosamines (N‐NAs); cancer; exposure; food; genotoxicity; margin of exposure (MOE); occurrence.
© 2023 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures



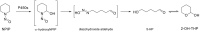




References
-
- Adami HO, Trolle Andersen I, Heide‐Jørgensen U, Chang ET, Nørgaard M and Toft Sørensen H, 2021. Ranitidine use and risk of upper gastrointestinal CancersRanitidine and upper gastrointestinal cancers. Cancer Epidemiology, Biomarkers and Prevention, 30, 2302–2308. - PubMed
-
- Adamson RH, 1989. Induction of hepatocellular carcinoma in nonhuman primates by chemical `carcinogens. Cancer Detection and Prevention, 14, 215–219. - PubMed
-
- Adamson RH and Sieber SM, 1979. The use of nonhuman primates for chemical carcinogenesis studies. Book: regulatory aspects of carcinogenesis and food additives: the Delaney clause. Ecotoxicology and Environmental Safety, 2, 275–302.
-
- Ahn HJ, Kim JH, Jo C, Lee CH and Byun MW, 2002a. Reduction of carcinogenic N‐Nitrosamines and residual nitrite in model system sausage by irradiation. Journal of Food Science, 67, 1370–1373.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
