Membrane phospholipid remodeling modulates nonalcoholic steatohepatitis progression by regulating mitochondrial homeostasis
- PMID: 36999536
- PMCID: PMC10544743
- DOI: 10.1097/HEP.0000000000000375
Membrane phospholipid remodeling modulates nonalcoholic steatohepatitis progression by regulating mitochondrial homeostasis
Abstract
Background and aims: NASH, characterized by inflammation and fibrosis, is emerging as a leading etiology of HCC. Lipidomics analyses in the liver have shown that the levels of polyunsaturated phosphatidylcholine (PC) are decreased in patients with NASH, but the roles of membrane PC composition in the pathogenesis of NASH have not been investigated. Lysophosphatidylcholine acyltransferase 3 (LPCAT3), a phospholipid (PL) remodeling enzyme that produces polyunsaturated PLs, is a major determinant of membrane PC content in the liver.
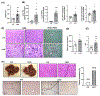
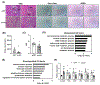
Approach and results: The expression of LPCAT3 and the correlation between its expression and NASH severity were analyzed in human patient samples. We examined the effect of Lpcat3 deficiency on NASH progression using Lpcat3 liver-specific knockout (LKO) mice. RNA sequencing, lipidomics, and metabolomics were performed in liver samples. Primary hepatocytes and hepatic cell lines were used for in vitro analyses. We showed that LPCAT3 was dramatically suppressed in human NASH livers, and its expression was inversely correlated with NAFLD activity score and fibrosis stage. Loss of Lpcat3 in mouse liver promotes both spontaneous and diet-induced NASH/HCC. Mechanistically, Lpcat3 deficiency enhances reactive oxygen species production due to impaired mitochondrial homeostasis. Loss of Lpcat3 increases inner mitochondrial membrane PL saturation and elevates stress-induced autophagy, resulting in reduced mitochondrial content and increased fragmentation. Furthermore, overexpression of Lpcat3 in the liver ameliorates inflammation and fibrosis of NASH.
Conclusions: These results demonstrate that membrane PL composition modulates the progression of NASH and that manipulating LPCAT3 expression could be an effective therapeutic for NASH.
Copyright © 2023 American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures
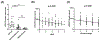





References
-
- Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018;68:268–279. - PubMed
-
- Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007;46:1081–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

