Hesperetin treatment attenuates glycation of lens proteins and advanced‑glycation end products generation
- PMID: 36999595
- PMCID: PMC10086570
- DOI: 10.3892/mmr.2023.12990
Hesperetin treatment attenuates glycation of lens proteins and advanced‑glycation end products generation
Abstract
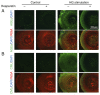
Advanced glycation end products (AGEs) in lens proteins increase with aging, thus inducing cataracts and/or presbyopia. Hesperetin (Hst), which is an abundant plant flavanone largely derived from citrus species, and its derivatives attenuate cataracts and presbyopia in vivo and in vitro; however, no reports have described its effects on AGE formation in lens proteins. The present study demonstrated that AGEs in lens proteins increase with age in mice. Additionally, it showed that Hst can prevent AGEs and N(ε)‑carboxymethyl‑lysine generation and modification of lens proteins using in vitro in human lens epithelial cell lines and ex vivo in mouse lens organ cultures. Furthermore, treatment with Hst prevented lens hardening and decreased chaperone activity in lens proteins. These results suggested that Hst and its derivatives are good candidates for the prevention of presbyopia and cataracts.
Keywords: advanced glycation end product; hesperetin; lens protein; natural flavonoid; presbyopia.
Conflict of interest statement
NM and SE are employees of Hayashibara Co., Ltd. (Okayama, Japan). The funders had no role in the design of the study; collection, analysis, or interpretation of data; writing of the manuscript; or decision to publish the results. The other authors also declare that they have no competing interests.
Figures




References
-
- Little MP, Kitahara CM, Cahoon EK, Bernier MO, Velazquez-Kronen R, Doody MM, Borrego D, Miller JS, Alexander BH, Simon SL, et al. Occupational radiation exposure and risk of cataract incidence in a cohort of US radiologic technologists. Eur J Epidemiol. 2018;33:1179–1191. doi: 10.1007/s10654-018-0435-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

