Real-world data on the efficacy and safety of immune-checkpoint inhibitors in elderly patients with non-small cell lung cancer
- PMID: 36999734
- PMCID: PMC10242309
- DOI: 10.1002/cam4.5889
Real-world data on the efficacy and safety of immune-checkpoint inhibitors in elderly patients with non-small cell lung cancer
Abstract
Purpose: Immune-checkpoint inhibitors (ICIs) are effective against advanced non-small cell lung cancer (NSCLC). However, whether the efficacy and safety of ICI treatment in elderly patients are similar to those in younger patients is unclear. This study was designed to address this question.
Methods: We enrolled patients who received ICI monotherapy in Japan between December 2015 and December 2017; those ≥75 years of age comprised the elderly group. We compared the efficacy and safety of ICI monotherapy in elderly patients with those in younger patients and explored prognostic factors in elderly patients.
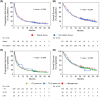
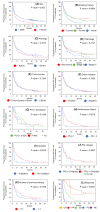
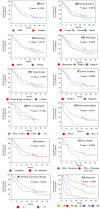
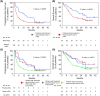
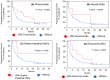
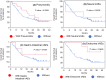
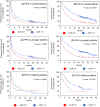
Results: We enrolled 676 patients; 137 (20.3%) were assigned to the elderly group. The median age of the elderly and younger groups was 78 (range, 75-85) and 66 (range, 34-74) years. The median progression-free survival (4.8 months vs. 3.3 months, p = 0.1589) and median overall survival (12.3 months vs. 13.0 months, p = 0.5587) were similar between the elderly and younger groups. Multivariate analysis revealed that a significantly better OS in the elderly group was associated with better responses to first- or second-line ICI treatment (p = 0.011) and more immune-related adverse events (irAEs) (p = 0.02). IrAEs that led to ICI discontinuation occurred in 34 of 137 patients (24.8%) in the elderly group, and their survival was significantly higher than that in those who did not have irAEs.
Conclusion: ICI is also effective in elderly NSCLC patients, and treatment discontinuation due to irAEs may be a good prognostic marker.
Keywords: elderly patient; immune-checkpoint inhibitor; non-small cell lung cancer.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Hajime Asahina reported receiving lecture fees from Chugai Pharmaceutical. Dr. Osamu Honjo reported lecture fees from Bristol‐Myers Squib K.K. Dr. Hisashi Tanaka reported receiving lecture fees from Chugai Pharmaceutical and Ono Pharmaceutical Co., Ltd during the conduct of the study. Dr. Hiroshi Yokouchi reported receiving lecture fees from AstraZeneca, and grants from Taiho Pharmaceutical, Sanofi, Bristol‐Myers Squibb, MSD, Takeda Pharmaceutical, Daiichi‐Sankyo, and Chugai Pharmaceutical during the conduct of the study. The other authors declare no conflicts of interest.
Figures

References
-
- Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non‐small‐cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23(28):6865‐6872. doi:10.1200/JCO.2005.02.527 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

