ITGA5 promotes tumor angiogenesis in cervical cancer
- PMID: 36999964
- PMCID: PMC10242342
- DOI: 10.1002/cam4.5873
ITGA5 promotes tumor angiogenesis in cervical cancer
Abstract
Purpose: Integrins are critical to cancer progression. Integrin alpha 5 (ITGA5) is correlated with the prognosis of cervical cancer patients. However, whether ITGA5 plays an active role in cervical cancer progression or not remains unknown.
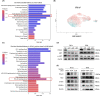
Methods: ITGA5 protein expression was detected in 155 human cervical cancer tissues by immunohistochemistry. Data from The Cancer Genome Atlas were utilized to identify risk factors for the overall survival of cervical cancer patients and ITGA5-associated differentially expressed genes. Analyses of single-cell RNA-seq based on Gene Expression Omnibus datasets were performed to show the coexpression of ITGA5 and angiogenesis factors. Tube formation assay, 3D spheroid sprout assay, qRT-PCR, Western Blotting, ELISA, and immunofluorescence were conducted to explore the angiogenic function of ITGA5 in vitro and underlying mechanisms.
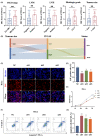
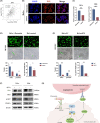
Results: High ITGA5 level was significantly correlated with increased risk in terms of overall survival and advanced disease stage in cervical cancer patients. ITGA5-associated differentially expressed genes linked ITGA5 to angiogenesis, and immunohistochemistry showed a positive correlation between ITGA5 and microvascular density in cervical cancer tissues. Moreover, tumor cells transfected with ITGA5-targeting siRNA decreased ability to promote endothelial tube formation in vitro. ITGA5/VEGFA coexpression was observed in a tumor cell subpopulation and the decreased endothelial angiogenesis by downregulating ITGA5 could be reversed by VEGFA. Bioinformatics analysis highlighted the PI3K-Akt signaling pathway as downstream of ITGA5. Downregulation of ITGA5 in tumor cells significantly decreased p-AKT and VEGFA levels. Fibronectin (FN1) coated cells or transfected with FN1-targeting siRNA showed fibronectin may play a critical role on ITGA5-mediated angiogenesis.
Conclusion: ITGA5 promotes angiogenesis and possibly be a potential predictive biomarker for poor survival of patients in cervical cancer.
Keywords: ITGA5; VEGFA; angiogenesis; cervical cancer.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Effects of Fibronectin 1 on Cell Proliferation, Senescence and Apoptosis of Human Glioma Cells Through the PI3K/AKT Signaling Pathway.Cell Physiol Biochem. 2018;48(3):1382-1396. doi: 10.1159/000492096. Epub 2018 Jul 26. Cell Physiol Biochem. 2018. Retraction in: Cell Physiol Biochem. 2020;54(6):1257. doi: 10.33594/000000317. PMID: 30048971 Retracted.
-
MicroRNA-29b Inhibits Angiogenesis by Targeting VEGFA through the MAPK/ERK and PI3K/Akt Signaling Pathways in Endometrial Carcinoma.Cell Physiol Biochem. 2017;41(3):933-946. doi: 10.1159/000460510. Epub 2017 Feb 20. Cell Physiol Biochem. 2017. PMID: 28222438
-
Triptolide inhibits angiogenesis in microvascular endothelial cells through regulation of miR-92a.J Physiol Biochem. 2019 Nov;75(4):573-583. doi: 10.1007/s13105-019-00707-2. Epub 2019 Nov 5. J Physiol Biochem. 2019. Retraction in: J Physiol Biochem. 2022 Aug;78(3):705. doi: 10.1007/s13105-022-00879-4. PMID: 31691162 Retracted.
-
ITGA5 induces mesenchymal transformation to promote gliomas progression via PI3K/AKT/mTORC1 signaling pathway.Sci Rep. 2025 Apr 19;15(1):13539. doi: 10.1038/s41598-025-98170-1. Sci Rep. 2025. PMID: 40253517 Free PMC article.
-
linc00958/miR-185-5p/RSF-1 modulates cisplatin resistance and angiogenesis through AKT1/GSK3β/VEGFA pathway in cervical cancer.Reprod Biol Endocrinol. 2022 Sep 2;20(1):132. doi: 10.1186/s12958-022-00995-2. Reprod Biol Endocrinol. 2022. PMID: 36056431 Free PMC article.
Cited by
-
Comprehensive Analysis of angiogenesis associated genes and tumor microenvironment infiltration characterization in cervical cancer.Heliyon. 2024 Jun 19;10(12):e33277. doi: 10.1016/j.heliyon.2024.e33277. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39021997 Free PMC article.
-
TGF-β-mediated activation of fibroblasts in cervical cancer: implications for tumor microenvironment and prognosis.PeerJ. 2025 Mar 19;13:e19072. doi: 10.7717/peerj.19072. eCollection 2025. PeerJ. 2025. PMID: 40124621 Free PMC article.
-
Characterization of macrophages in head and neck squamous cell carcinoma and development of MRG-based risk signature.Sci Rep. 2024 Apr 30;14(1):9914. doi: 10.1038/s41598-024-60516-6. Sci Rep. 2024. PMID: 38688945 Free PMC article.
-
Development and validation of a prognostic model for efferocytosis-associated genes in cervical squamous cell carcinoma.Discov Oncol. 2025 Jul 1;16(1):1224. doi: 10.1007/s12672-025-02901-9. Discov Oncol. 2025. PMID: 40591190 Free PMC article.
-
Immunological role and prognostic value of ITGA3 and ITGA5 in oral squamous cell carcinoma.Sci Rep. 2025 Aug 17;15(1):30046. doi: 10.1038/s41598-025-16026-0. Sci Rep. 2025. PMID: 40820179 Free PMC article.

