Safety and Efficacy of Axicabtagene Ciloleucel versus Standard of Care in Patients 65 Years of Age or Older with Relapsed/Refractory Large B-Cell Lymphoma
- PMID: 36999993
- PMCID: PMC10183830
- DOI: 10.1158/1078-0432.CCR-22-3136
Safety and Efficacy of Axicabtagene Ciloleucel versus Standard of Care in Patients 65 Years of Age or Older with Relapsed/Refractory Large B-Cell Lymphoma
Abstract
Purpose: Older patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) may be considered ineligible for curative-intent therapy including high-dose chemotherapy with autologous stem-cell transplantation (HDT-ASCT). Here, we report outcomes of a preplanned subgroup analysis of patients ≥65 years in ZUMA-7.
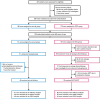
Patients and methods: Patients with LBCL refractory to or relapsed ≤12 months after first-line chemoimmunotherapy were randomized 1:1 to axicabtagene ciloleucel [axi-cel; autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy] or standard of care (SOC; 2-3 cycles of chemoimmunotherapy followed by HDT-ASCT). The primary endpoint was event-free survival (EFS). Secondary endpoints included safety and patient-reported outcomes (PROs).
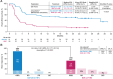
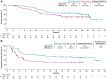
Results: Fifty-one and 58 patients aged ≥65 years were randomized to axi-cel and SOC, respectively. Median EFS was greater with axi-cel versus SOC (21.5 vs. 2.5 months; median follow-up: 24.3 months; HR, 0.276; descriptive P < 0.0001). Objective response rate was higher with axi-cel versus SOC (88% vs. 52%; OR, 8.81; descriptive P < 0.0001; complete response rate: 75% vs. 33%). Grade ≥3 adverse events occurred in 94% of axi-cel and 82% of SOC patients. No grade 5 cytokine release syndrome or neurologic events occurred. In the quality-of-life analysis, the mean change in PRO scores from baseline at days 100 and 150 favored axi-cel for EORTC QLQ-C30 Global Health, Physical Functioning, and EQ-5D-5L visual analog scale (descriptive P < 0.05). CAR T-cell expansion and baseline serum inflammatory profile were comparable in patients ≥65 and <65 years.
Conclusions: Axi-cel is an effective second-line curative-intent therapy with a manageable safety profile and improved PROs for patients ≥65 years with R/R LBCL.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
- 1078-0432. doi: 10.1158/1078-0432.CCR-29-10-HI doi: 10.1158/1078-0432.CCR-29-10-HI
References
-
- Surveillance Epidemiology and End Results. Cancer Stat Facts: NHL — Diffuse Large B-Cell Lymphoma (DLBCL); 2022. Available from: https://seer.cancer.gov/statfacts/html/dlbcl.html.
-
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:498–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

