Pazopanib with Topotecan weekly for patients with platinum-resistant or intermediate-sensitive recurrent ovarian cancer: results of a multicentre, open label phase I/II study (TOPAZ)
- PMID: 37000264
- PMCID: PMC10374680
- DOI: 10.1007/s00432-023-04647-9
Pazopanib with Topotecan weekly for patients with platinum-resistant or intermediate-sensitive recurrent ovarian cancer: results of a multicentre, open label phase I/II study (TOPAZ)
Abstract
Purpose: Pazopanib has promising antiangiogenetic activity in solid cancers. The investigator-initiated phase I/II trial evaluated the combination of Topotecan with Pazopanib in platinum-resistant or intermediate-sensitive recurrent ovarian cancer (ROC).
Methods: Patients (≥ 18 years) with first or second recurrence were enrolled in this multicentre open-label trial. Phase I analysed Topotecan 4 mg/m2 (day 1, 8, 15, ever 28 days) for six cycles to identify the maximum tolerated dose (MTD) of Pazopanib added in a dose-escalating scheme with 400 mg starting dose. The phase II analysed safety and efficacy aspects. For all patients with clinical remission a maintenance with Pazopanib until progression was allowed. This trial is registered with ClinicalTrials.gov, number NCT01600573.
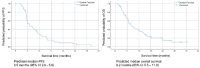
Results: Between June 2012 and February 2017, 11 patients were enrolled in the phase I, and 50 patients in the phase II study. The MTD of Pazopanib was determined by 400 mg/daily. Haematological and liver toxicities determined the dose limiting toxicities (DLT) and the most common grade 3-4 adverse events: leucopenia (25%), neutropenia (22%), thrombocytopenia (19%), accumulation of cholestatic (20%) and hepatocellular damage (15%), which often caused dose modifications, but no new life-threatening events. Overall response was 16% and clinical benefit rate 68%. Median progression-free survival (PFS) was 3.5 months (95% CI 2.0-5.0). Due to early progression only 20% of the patients were able to start with maintenance treatment.
Conclusion: The combination of pazopanib and weekly topotecan is feasible, resulting in a manageable haematological and liver toxicity, but despite its encouraging response rate, was not associated with a significant survival benefit.
Keywords: Pazopanib; Platinum-resistant ovarian cancer; Topotecan.
© 2023. The Author(s).
Conflict of interest statement
We declare the following potential
Figures
References
-
- Boudou-Rouquette P et al (2016) Clinical pharmacology, drug-drug interactions and safety of pazopanib: a review. Expert Opin Drug Metab Toxicol 12(12):1433–1444 - PubMed
-
- Chase DM, Chaplin DJ, Monk BJ (2017) The development and use of vascular targeted therapy in ovarian cancer. Gynecol Oncol 145(2):393–406 - PubMed
-
- Chekerov R et al (2015) Treosulfan in the treatment of advanced ovarian cancer - results of a german multicenter non-interventional study. Anticancer Res 35(12):6869–6875 - PubMed
-
- Chekerov R et al (2018) Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 19(9):1247–1258 - PubMed
-
- du Bois, A., et al. 2013 AGO-OVAR 12: A randomized placebo-controlled GCIG/ENGOT-Intergroup Phase III Trial of Standard Frontline Chemotherapy +/- Nintedanib for Advanced Ovarian Cancer . International Journal of Gynecological Cancer. 238, Supplement 1).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical