Cardiovascular outcomes in patients with atrial fibrillation concomitantly treated with antiarrhythmic drugs and non-vitamin k antagonist oral anticoagulants
- PMID: 37000581
- PMCID: PMC10227765
- DOI: 10.1093/europace/euad083
Cardiovascular outcomes in patients with atrial fibrillation concomitantly treated with antiarrhythmic drugs and non-vitamin k antagonist oral anticoagulants
Abstract
Aims: Limited data compared antiarrhythmic drugs (AADs) with concomitant non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients, hence the aim of the study.
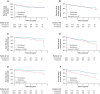
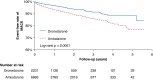
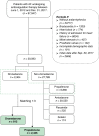
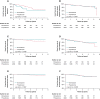
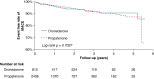
Methods and results: National health insurance database were retrieved during 2012-17 for study. We excluded patients not taking AADs, bradycardia, heart block, heart failure admission, mitral stenosis, prosthetic valve, incomplete demographic data, and follow-up <3 months. Outcomes were compared in Protocol 1, dronedarone vs. non-dronedarone; Protocol 2, dronedarone vs. amiodarone; and Protocol 3, dronedarone vs. propafenone. Outcomes were acute myocardial infarction (AMI), ischaemic stroke/systemic embolism, intracranial haemorrhage (ICH), major bleeding, cardiovascular death, all-cause mortality, and major adverse cardiovascular event (MACE) (including AMI, ischaemic stroke, and cardiovascular death). In Protocol 1, 2298 dronedarone users and 6984 non-dronedarone users (amiodarone = 4844; propafenone = 1914; flecainide = 75; sotalol = 61) were analysed. Dronedarone was associated with lower ICH (HR = 0.61, 95% CI = 0.38-0.99, P = 0.0436), cardiovascular death (HR = 0.24, 95% CI = 0.16-0.37, P < 0.0001), all-cause mortality (HR = 0.33, 95% CI = 0.27-0.42, P < 0.0001), and MACE (HR = 0.56, 95% CI = 0.45-0.70, P < 0.0001). In Protocol 2, 2231 dronedarone users and 6693 amiodarone users were analysed. Dronedarone was associated with significantly lower ICH (HR = 0.53, 95%=CI 0.33-0.84, P = 0.0078), cardiovascular death (HR = 0.20, 95% CI = 0.13-0.31, P < 0.0001), all-cause mortality (HR 0.27, 95% CI 0.22-0.34, P < 0.0001), and MACE (HR = 0.53, 95% CI = 0.43-0.66, P < 0.0001), compared with amiodarone. In Protocol 3, 812 dronedarone users and 2436 propafenone users were analysed. There were no differences between two drugs for primary and secondary outcomes.
Conclusion: The use of dronedarone with NOACs was associated with cardiovascular benefits in an Asian population, compared with non-dronedarone AADs and amiodarone.
Keywords: Amiodarone; Atrial fibrillation; Dronedarone; Non-vitamin K antagonist oral anticoagulants; Propafenone.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: This investigator-sponsored study received funding from Sanofi. Victor Chien-Chia Wu, Chun-Li Wang, and Shang-Hung Chang received grants and support from Sanofi. The remaining authors have nothing to disclose.
Figures




References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. - PubMed
-
- Boriani G, Blomström-Lundqvist C, Hohnloser SH, Bergfeldt L, Botto GL, Capucci A, et al. . Safety and efficacy of dronedarone from clinical trials to real-world evidence: implications for its use in atrial fibrillation. Europace 2019;21:1764–75. - PubMed
-
- Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. . Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–78. - PubMed
-
- Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, et al. . Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007;357:987–99. - PubMed
-
- Kober L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, et al. . Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008;358:2678–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

