Arterial pulse wave modeling and analysis for vascular-age studies: a review from VascAgeNet
- PMID: 37000606
- PMCID: PMC7614613
- DOI: 10.1152/ajpheart.00705.2022
Arterial pulse wave modeling and analysis for vascular-age studies: a review from VascAgeNet
Abstract
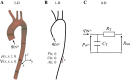
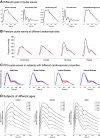
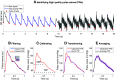
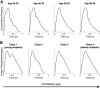
Arterial pulse waves (PWs) such as blood pressure and photoplethysmogram (PPG) signals contain a wealth of information on the cardiovascular (CV) system that can be exploited to assess vascular age and identify individuals at elevated CV risk. We review the possibilities, limitations, complementarity, and differences of reduced-order, biophysical models of arterial PW propagation, as well as theoretical and empirical methods for analyzing PW signals and extracting clinically relevant information for vascular age assessment. We provide detailed mathematical derivations of these models and theoretical methods, showing how they are related to each other. Finally, we outline directions for future research to realize the potential of modeling and analysis of PW signals for accurate assessment of vascular age in both the clinic and in daily life.
Keywords: aging; arteriosclerosis; atherosclerosis; hemodynamics; pulse wave.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


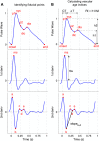
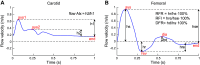
References
-
- Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo A-P, Hering D, López-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 388: 2665–2712, 2016. doi:10.1016/S0140-6736(16)31134-5. - DOI - PubMed
-
- Climie RE, Alastruey J, Mayer CC, Schwarz A, Laucyte-Cibulskiene A, Voicehovska J, Bianchini E, Bruno R-M, Charlton PH, Grillo A, Guala A, Hallab M, Hametner B, Jankowski P, Königstein K, Lebedeva A, Mozos I, Pucci G, Puzantian H, Terentes-Printzios D, Yetik-Anacak G, Park C, Nilsson PM, Weber T. Vascular ageing—moving from bench towards bedside. Eur J Prev Cardiol. 2023 May 08 [Epub ahead of print]. doi:10.1093/eurjpc/zwad028. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

