The ovaries of transgender men indicate effects of high dose testosterone on the primordial and early growing follicle pool
- PMID: 37000633
- PMCID: PMC10160535
- DOI: 10.1530/RAF-22-0102
The ovaries of transgender men indicate effects of high dose testosterone on the primordial and early growing follicle pool
Abstract
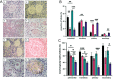
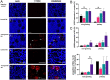
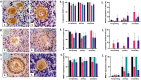
Androgens are essential in normal ovarian function and follicle health but hyperandrogenism, as seen in polycystic ovary syndrome, is associated with disordered follicle development. There are few data on the effect of long-term exposure to high levels of testosterone as found in transgender men receiving gender-affirming endocrine therapy. In this study, we investigate the effect of testosterone on the development, morphological health and DNA damage and repair capacity of human ovarian follicles in vivo and their survival in vitro. Whole ovaries were obtained from transgender men (mean age: 27.6 ± 1.7 years; range 20-34 years, n = 8) at oophorectomy taking pre-operative testosterone therapy. This was compared to cortical biopsies from age-matched healthy women obtained at caesarean section (mean age: 31.8±1.5 years; range= 25-35 years, n=8). Cortical tissues were dissected into fragments and either immediately fixed for histological analysis or cultured for 6 days and subsequently fixed. Follicle classification and morphological health were evaluated from histological sections stained with H&E and expression of γH2AX as a marker of DNA damage by IHC. In uncultured tissue, testosterone exposure was associated with reduced follicle growth activation, poor follicle health and increased DNA damage. After 6 days of culture, there was enhanced follicle activation compared to control with further deterioration in morphological health and increased DNA damage. These data indicate that high circulating concentrations of testosterone have effects on the primordial and small-growing follicles of the ovary. These results may have implications for transgender men receiving gender-affirming therapy prior to considering pregnancy or fertility preservation measures.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

