Proteolytic processing of galectin-3 by meprin metalloproteases is crucial for host-microbiome homeostasis
- PMID: 37000885
- PMCID: PMC10065446
- DOI: 10.1126/sciadv.adf4055
Proteolytic processing of galectin-3 by meprin metalloproteases is crucial for host-microbiome homeostasis
Abstract
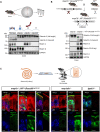
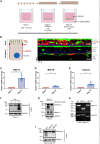
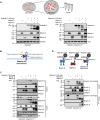
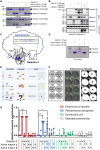
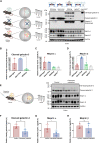
The metalloproteases meprin α and meprin β are highly expressed in the healthy gut but significantly decreased in inflammatory bowel disease, implicating a protective role in mucosal homeostasis. In the colon, meprin α and meprin β form covalently linked heterodimers tethering meprin α to the plasma membrane, therefore presenting dual proteolytic activity in a unique enzyme complex. To unravel its function, we applied N-terminomics and identified galectin-3 as the major intestinal substrate for meprin α/β heterodimers. Galectin-3-deficient and meprin α/β double knockout mice show similar alterations in their microbiome in comparison to wild-type mice. We further demonstrate that meprin α/β heterodimers differentially process galectin-3 upon bacterial infection, in germ-free, conventionally housed (specific pathogen-free), or wildling mice, which in turn regulates the bacterial agglutination properties of galectin-3. Thus, the constitutive cleavage of galectin-3 by meprin α/β heterodimers may play a key role in colon host-microbiome homeostasis.
Figures



References
-
- A. J. Macpherson, E. Slack, M. B. Geuking, K. D. McCoy, The mucosal firewalls against commensal intestinal microbes. Semin. Immunopathol. 31, 145–149 (2009). - PubMed
-
- M. E. Johansson, J. K. Gustafsson, J. Holmén-Larsson, K. S. Jabbar, L. Xia, H. Xu, F. K. Ghishan, F. A. Carvalho, A. T. Gewirtz, H. Sjövall, G. C. Hansson, Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

