Synthesis and Characterization of Phenylalanine Amides Active against Mycobacterium abscessus and Other Mycobacteria
- PMID: 37001025
- PMCID: PMC10586324
- DOI: 10.1021/acs.jmedchem.3c00009
Synthesis and Characterization of Phenylalanine Amides Active against Mycobacterium abscessus and Other Mycobacteria
Abstract
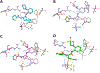
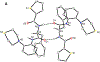
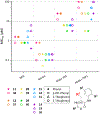
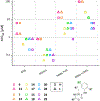
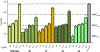
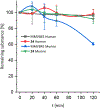
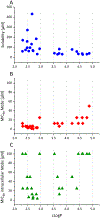
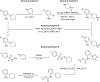
Nα-2-thiophenoyl-d-phenylalanine-2-morpholinoanilide [MMV688845, Pathogen Box; Medicines for Malaria Venture; IUPAC: (2R)-N-(1-((2-morpholinophenyl)amino)-1-oxo-3-phenylpropan-2-yl)thiophene-2-carboxamide)] is a hit compound, which shows activity against Mycobacterium abscessus (MIC90 6.25-12.5 μM) and other mycobacteria. This work describes derivatization of MMV688845 by introducing a thiomorpholine moiety and the preparation of the corresponding sulfones and sulfoxides. The molecular structures of three analogs are confirmed by X-ray crystallography. Conservation of the essential R configuration during synthesis is proven by chiral HPLC for an exemplary compound. All analogs were characterized in a MIC assay against M. abscessus, Mycobacterium intracellulare, Mycobacterium smegmatis, and Mycobacterium tuberculosis. The sulfone derivatives exhibit lower MIC90 values (M. abscessus: 0.78 μM), and the sulfoxides show higher aqueous solubility than the hit compound. The most potent derivatives possess bactericidal activity (99% inactivation of M. abscessus at 12.5 μM), while they are not cytotoxic against mammalian cell lines.
Figures



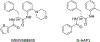



Similar articles
-
In Vitro Profiling of the Synthetic RNA Polymerase Inhibitor MMV688845 against Mycobacterium abscessus.Microbiol Spectr. 2022 Dec 21;10(6):e0276022. doi: 10.1128/spectrum.02760-22. Epub 2022 Nov 15. Microbiol Spectr. 2022. PMID: 36377951 Free PMC article.
-
Racemization-free synthesis of Nα-2-thiophenoyl-phenylalanine-2-morpholinoanilide enantiomers and their antimycobacterial activity.Amino Acids. 2021 Aug;53(8):1187-1196. doi: 10.1007/s00726-021-03044-1. Epub 2021 Jul 14. Amino Acids. 2021. PMID: 34259925 Free PMC article.
-
Mycobacterium tuberculosis DprE1 Inhibitor OPC-167832 Is Active against Mycobacterium abscessus In Vitro.Antimicrob Agents Chemother. 2022 Dec 20;66(12):e0123722. doi: 10.1128/aac.01237-22. Epub 2022 Nov 9. Antimicrob Agents Chemother. 2022. PMID: 36350151 Free PMC article.
-
[A pharmacologic approach to treatment of Mycobacterium abscessus pulmonary disease].Rev Mal Respir. 2024 Jan;41(1):29-42. doi: 10.1016/j.rmr.2023.10.010. Epub 2023 Nov 27. Rev Mal Respir. 2024. PMID: 38016833 Review. French.
-
A review of current and promising nontuberculous mycobacteria antibiotics.Future Med Chem. 2021 Aug;13(16):1367-1395. doi: 10.4155/fmc-2021-0048. Epub 2021 Jun 24. Future Med Chem. 2021. PMID: 34165325 Review.
Cited by
-
A Simple In Vitro Method to Determine Bactericidal Activity Against Mycobacterium abscessus Under Hypoxic Conditions.Antibiotics (Basel). 2025 Mar 13;14(3):299. doi: 10.3390/antibiotics14030299. Antibiotics (Basel). 2025. PMID: 40149109 Free PMC article.
-
3-[(Benzo-1,3-dioxol-5-yl)amino]-4-methoxycyclobut-3-ene-1,2-dione: polymorphism and twinning of a precursor to an antimycobacterial squaramide.Acta Crystallogr C Struct Chem. 2024 Aug 1;80(Pt 8):375-382. doi: 10.1107/S2053229624006211. Epub 2024 Jul 5. Acta Crystallogr C Struct Chem. 2024. PMID: 38967633 Free PMC article.
-
Broad-Spectrum In Vitro Activity of Nα-Aroyl-N-Aryl-Phenylalanine Amides against Non-Tuberculous Mycobacteria and Comparative Analysis of RNA Polymerases.Antibiotics (Basel). 2024 Apr 28;13(5):404. doi: 10.3390/antibiotics13050404. Antibiotics (Basel). 2024. PMID: 38786132 Free PMC article.
-
Toward better cures for Mycobacterium abscessus lung disease.Clin Microbiol Rev. 2024 Dec 10;37(4):e0008023. doi: 10.1128/cmr.00080-23. Epub 2024 Oct 3. Clin Microbiol Rev. 2024. PMID: 39360834 Review.
-
Synthesis and in vitro Metabolic Stability of Sterically Shielded Antimycobacterial Phenylalanine Amides.ChemMedChem. 2024 Mar 15;19(6):e202300593. doi: 10.1002/cmdc.202300593. Epub 2024 Feb 29. ChemMedChem. 2024. PMID: 38329388 Free PMC article.
References
-
- World Health Organization. Module 4: Treatment Drug-Susceptible Tuberculosis Treatment; World Health Organization, 2022. https://www.who.int/publications/i/item/9789240048126 (accessed 2023-02-21).
-
- World Health Organization. Global Tuberculosis Report 2022; 2022. https://www.who.int/publications/i/item/9789240061729 (accessed 2023-01-02).
-
- Sharma A; de Rosa M; Singla N; Singh G; Barnwal RP; Pandey A Tuberculosis: An Overview of the Immunogenic Response, Disease Progression, and Medicinal Chemistry Efforts in the Last Decade toward the Development of Potential Drugs for Extensively Drug-Resistant Tuberculosis Strains. J Med Chem 2021, 64 (8), 4359–4395. 10.1021/ACS.JMEDCHEM.0C01833. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

