The causal effects of lipid traits on kidney function in Africans: bidirectional and multivariable Mendelian-randomization study
- PMID: 37001235
- PMCID: PMC10070509
- DOI: 10.1016/j.ebiom.2023.104537
The causal effects of lipid traits on kidney function in Africans: bidirectional and multivariable Mendelian-randomization study
Abstract
Background: Observational studies have investigated the effect of serum lipids on kidney function, but these findings are limited by confounding, reverse causation and have reported conflicting results. Mendelian randomization (MR) studies address this confounding problem. However, they have been conducted mostly in European ancestry individuals. We, therefore, set out to investigate the effect of lipid traits on the estimated glomerular filtration rate (eGFR) based on serum creatinine in individuals of African ancestry.
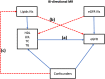
Methods: We used the two-sample and multivariable Mendelian randomization (MVMR) approaches; in which instrument variables (IV's) for the predictor (lipid traits) were derived from summary-level data of a meta-analyzed African lipid GWAS (MALG, n = 24,215) from the African Partnership for Chronic Disease Research (APCDR) (n = 13,612) & the Africa Wits-IN-DEPTH partnership for Genomics studies (AWI-Gen) dataset (n = 10,603). The outcome IV's were computed from the eGFR summary-level data of African-ancestry individuals within the Million Veteran Program (n = 57,336). A random-effects inverse variance method was used in our primary analysis, and pleiotropy was adjusted for using robust and penalized sensitivity testing. The lipid predictors for the MVMR were high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides (TG).
Findings: We found a significant causal association between genetically predicted low-density lipoprotein (LDL) cholesterol and eGFR in African ancestry individuals β = 1.1 (95% CI [0.411-1.788]; p = 0.002). Similarly, total cholesterol (TC) showed a significant causal effect on eGFR β = 1.619 (95% CI [0.412-2.826]; p = 0.009). However, the IVW estimate showed that genetically predicted HDL-C β = -0.164, (95% CI = [-1.329 to 1.00]; p = 0.782), and TG β = -0.934 (CI = [-2.815 to 0.947]; p = 0.33) were not significantly causally associated with the risk of eGFR. In the multivariable analysis inverse-variance weighted (MVIVW) method, there was evidence for a causal association between LDL and eGFR β = 1.228 (CI = [0.477-1.979]; p = 0.001). A significant causal effect of Triglycerides (TG) on eGFR in the MVIVW analysis β = -1.3 ([-2.533 to -0.067]; p = 0.039) was observed as well. All the causal estimates reported reflect a unit change in the outcome per a 1 SD increase in the exposure. HDL showed no evidence of a significant causal association with eGFR in the MVIVW method (β = -0.117 (95% CI [-1.252 to 0.018]; p = 0.840)). We found no evidence of a reverse causal impact of eGFR on serum lipids. All our sensitivity analyses indicated no strong evidence of pleiotropy or heterogeneity between our instrumental variables for both the forward and reverse MR analysis.
Interpretation: In this African ancestry population, genetically predicted higher LDL-C and TC are causally associated with higher eGFR levels, which may suggest that the relationship between LDL, TC and kidney function may be U-shaped. And as such, lowering LDL_C does not necessarily improve risk of kidney disease. This may also imply the reason why LDL_C is seen to be a poorer predictor of kidney function compared to HDL. In addition, this further supports that more work is warranted to confirm the potential association between lipid traits and risk of kidney disease in individuals of African Ancestry.
Funding: Wellcome (220740/Z/20/Z).
Keywords: Chronic kidney disease; Kidney function; Serum lipids; Two-sample Mendelian Randomization; eGFR.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests DG is employed part-time by Novo Nordisk and has received consultancy fees from Policy Wisdom. No potential conflicts of interest relevant to this article were reported by all other authors.
Figures


References
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150. - PubMed
-
- Webster A.C., Nagler E.V., Morton R.L., Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–1252. - PubMed
-
- Schaeffner E.S., Kurth T., Curhan G.C., et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–2091. - PubMed
-
- Cases A., Coll E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl. 2005;99:S87–S93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

