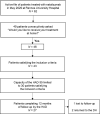
Switching from natalizumab administration at the day hospital to administration at home. A 1 year prospective study of patient experience and quality of life in 30 consecutive patients with multiple sclerosis (TYSAD-35)
- PMID: 37001411
- PMCID: PMC10049890
- DOI: 10.1016/j.msard.2023.104657
Switching from natalizumab administration at the day hospital to administration at home. A 1 year prospective study of patient experience and quality of life in 30 consecutive patients with multiple sclerosis (TYSAD-35)
Abstract
Background: In the context of the COVID-19 pandemic, French health authorities allowed the home administration of natalizumab by a healthcare-at-home service. We evaluated the patients' perception of care quality following the transition from day-hospital to home natalizumab administration.
Methods: Thirty relapsing-remitting multiple sclerosis (MS) patients treated with natalizumab were prospectively evaluated for one year after changing onto a home treatment procedure, using MusiCare, the first MS-specific questionnaire to evaluate patient experience and MusiQol. A numerical rating scale score for satisfaction and a dedicated questionnaire concerning patient experience were completed after each infusion. The primary endpoint was the mean difference in MusiCare score between baseline and 12 months.
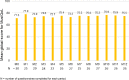
Results: From June 2020 to November 2021, 306 infusions were performed at home. Three patients withdrew from the study (one lost to follow-up and two preferred to return at the day hospital). No worsening of patient experience or quality of life was observed. The mean scores of the Musicare dimensions were higher at 12 months than at baseline, significantly for the "relationship with healthcare professionals" (p = 0.0203). The MusiQol global score remained stable but the coping and friendship dimensions were significantly better at M12 than at baseline (p = 0.0491 and p = 0.0478, respectively). The satisfaction questionnaire highlighted some pain during the infusions (21.8%) and contradictions between healthcare professionals (17.2%). The mean score for satisfaction with care was 9.1/10. No safety concerns were identified.
Conclusion: The positive experience of patients with home natalizumab administration provides an important opportunity to improve the quality of patient care.
Keywords: Multiple sclerosis, Quality of life, Home infusion therapy, Natalizumab; Patient experience; administration & dosage, Patient-centered care.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. E. Le Page received honoraria for consulting or lectures, invitations for national and international congresses from Biogen, Merck, Teva, Sanofi-Genzyme, Novartis, Alexion, Roche; research support from Teva and Biogen; academic research grants from PHRC and LFSEP, and travel grant from ARSEP Foundation. H. Doyen received honoraria for consulting from Biogen. L. Michel received honoraria for consulting from sanofi, roche, Janssen, celgene, Merck and novartis. S. Lamy, D.Veillard, A. Kerbrat, E. Chretien, A. Ousmen and G. Edan reports no disclosures.
Figures
References
-
- Butzkueven H., Kappos L., Wiendl H., et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP) J. Neurol. Neurosurg. Psychiatry. 2020;91(6):660–668. doi: 10.1136/jnnp-2019-322326. - DOI - PMC - PubMed
-
- Committee on Quality of Health Care in America. Crossing the Quality Chasm: (317382004-001). doi:10.1037/e317382004-001. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical