Performance of antigen lateral flow devices in the UK during the alpha, delta, and omicron waves of the SARS-CoV-2 pandemic: a diagnostic and observational study
- PMID: 37001541
- PMCID: PMC10048397
- DOI: 10.1016/S1473-3099(23)00129-9
Performance of antigen lateral flow devices in the UK during the alpha, delta, and omicron waves of the SARS-CoV-2 pandemic: a diagnostic and observational study
Abstract
Background: Antigen lateral flow devices (LFDs) have been widely used to control SARS-CoV-2. We aimed to improve understanding of LFD performance with changes in variant infections, vaccination, viral load, and LFD use, and in the detection of infectious individuals.
Methods: In this diagnostic study, paired LFD and RT-PCR test results were prospectively collected from asymptomatic and symptomatic participants in the UK between Nov 4, 2020, and March 21, 2022, to support the National Health Service (NHS) England's Test and Trace programme. The LFDs evaluated were the Innova SARS-CoV-2 Antigen Rapid Qualitative Test, the Orient Gene Rapid Covid-19 (Antigen) Self-Test, and the Acon Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing). Test results were collected across various community testing settings, including predeployment testing sites, routine testing centres, homes, schools, universities, workplaces, targeted community testing, and from health-care workers. We used multivariable logistic regression to analyse LFD sensitivity and specificity using RT-PCR as a reference standard, adjusting for viral load, LFD manufacturer, test setting, age, sex, test assistance, symptom status, vaccination status, and SARS-CoV-2 variant. National contact tracing data from NHS Test and Trace (Jan 1, 2021, to Jan 11, 2022) were used to estimate the proportion of transmitting index patients (with ≥1 RT-PCR-positive or LFD-positive contact) potentially detectable by LFDs (specifically Innova, as the most widely used LFD) with time, accounting for index viral load, variant, and symptom status.
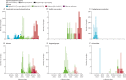
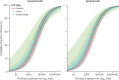
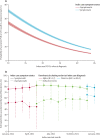
Findings: We assessed 75 382 pairs of LFD and RT-PCR tests. Of these, 4131 (5·5%) were RT-PCR-positive. LFD sensitivity versus RT-PCR was 63·2% (95% CI 61·7-64·6) and specificity was 99·71% (95% CI 99·66-99·74). Increased viral load was independently associated with being LFD positive (adjusted odds ratio [aOR] 2·85 [95% CI 2·66-3·06] per 1 log10 copies per mL increase; p<0·0001). There was no evidence that LFD sensitivity differed for delta (B.1.617.2) infections versus alpha (B.1.1.7) or pre-alpha (B.1.177) infections (aOR 1·00 [0·69-1·45]; p=0·99), whereas omicron (BA.1 or BA.2) infections appeared more likely to be LFD positive (aOR 1·63 [1·02-2·59]; p=0·042). Sensitivity was higher in symptomatic participants (68·7% [95% CI 66·9-70·4]) than in asymptomatic participants (52·8% [50·1-55·4]). Among 347 374 unique index patients with probable onward transmission, 78·3% (95% CI 75·3-81·2) were estimated to have been detectable with LFDs (Innova), and this proportion was mostly stable with time and for successive variants. Overall, the estimated proportion of infectious index patients detectable by the Innova LFD was lower in asymptomatic patients (57·6% [53·6-61·9]) versus symptomatic patients (79·7% [76·7-82·5]).
Interpretation: LFDs remained able to detect most SARS-CoV-2 infections throughout vaccine roll-out and across different viral variants. LFDs can potentially detect most infections that transmit to others and reduce the risk of transmission. However, performance is lower in asymptomatic individuals than in symptomatic individuals.
Funding: UK Health Security Agency, the UK Government Department of Health and Social Care, National Institute for Health Research (NIHR) Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance, and the University of Oxford NIHR Biomedical Research Centre.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
The performance of rapid antigen tests against SARS-CoV-2 variants.Lancet Infect Dis. 2023 Aug;23(8):883-884. doi: 10.1016/S1473-3099(23)00186-X. Epub 2023 Mar 28. Lancet Infect Dis. 2023. PMID: 37001540 No abstract available.
Similar articles
-
Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: a single-centre laboratory evaluation study.Lancet Microbe. 2021 Sep;2(9):e461-e471. doi: 10.1016/S2666-5247(21)00143-9. Epub 2021 Jun 30. Lancet Microbe. 2021. PMID: 34226893 Free PMC article.
-
Rapid antigen testing for SARS-CoV-2 by lateral flow assay: A field evaluation of self- and professional testing at UK community testing sites.J Clin Virol. 2024 Apr;171:105654. doi: 10.1016/j.jcv.2024.105654. Epub 2024 Feb 15. J Clin Virol. 2024. PMID: 38387136
-
COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing.EClinicalMedicine. 2021 Jun;36:100924. doi: 10.1016/j.eclinm.2021.100924. Epub 2021 May 30. EClinicalMedicine. 2021. PMID: 34101770 Free PMC article.
-
A systematic review of the sensitivity and specificity of lateral flow devices in the detection of SARS-CoV-2.BMC Infect Dis. 2021 Aug 18;21(1):828. doi: 10.1186/s12879-021-06528-3. BMC Infect Dis. 2021. PMID: 34407759 Free PMC article.
-
Accuracy of rapid point-of-care antigen-based diagnostics for SARS-CoV-2: An updated systematic review and meta-analysis with meta-regression analyzing influencing factors.PLoS Med. 2022 May 26;19(5):e1004011. doi: 10.1371/journal.pmed.1004011. eCollection 2022 May. PLoS Med. 2022. PMID: 35617375 Free PMC article.
Cited by
-
A population-based study of the trend in SARS-CoV-2 diagnostic modalities from the beginning of the pandemic to the Omicron surge in Kyoto City, Kyoto, Japan.BMC Public Health. 2023 Dec 21;23(1):2551. doi: 10.1186/s12889-023-17498-3. BMC Public Health. 2023. PMID: 38129830 Free PMC article.
-
Effectiveness and user experience of nose and throat swabbing techniques for SARS-CoV-2 detection: results from the UK COVID-19 National Testing Programme.BMC Glob Public Health. 2025 Jan 13;3(1):5. doi: 10.1186/s44263-024-00121-x. BMC Glob Public Health. 2025. PMID: 39806484 Free PMC article.
-
Sensitivity of rapid antigen tests against SARS-CoV-2 Omicron and Delta variants.J Clin Microbiol. 2023 Oct 24;61(10):e0013823. doi: 10.1128/jcm.00138-23. Epub 2023 Sep 20. J Clin Microbiol. 2023. PMID: 37728336 Free PMC article.
-
Diagnostic performance of rapid antigen tests (RAT) for COVID-19 and factors associated with RAT-negative results among RT-PCR-positive individuals during Omicron BA.2, BA.5 and XBB.1 predominance.BMC Infect Dis. 2024 May 21;24(1):504. doi: 10.1186/s12879-024-09408-8. BMC Infect Dis. 2024. PMID: 38769524 Free PMC article.
-
Continuous false positive results by SARS-CoV-2 rapid antigen testing: a case report.Front Public Health. 2023 Nov 3;11:1240308. doi: 10.3389/fpubh.2023.1240308. eCollection 2023. Front Public Health. 2023. PMID: 38026284 Free PMC article.
References
-
- Mahase E. Covid-19: UK regulator approves lateral flow test for home use despite accuracy concerns. BMJ. 2020;371 - PubMed
-
- NHS England NHS England and NHS Improvement rollout of lateral flow devices for asymptomatic staff testing for SARS CoV-2 (phase 2: trusts) Nov 16, 2020. https://www.england.nhs.uk/coronavirus/documents/nhs-england-and-nhs-imp...
-
- Deeks J, Raffle A, Gill M. Covid-19: government must urgently rethink lateral flow test roll out. Jan 12, 2023. https://blogs.bmj.com/bmj/2021/01/12/covid-19-government-must-urgently-r...
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

