Efficacy and safety of emapalumab in macrophage activation syndrome
- PMID: 37001971
- PMCID: PMC10314091
- DOI: 10.1136/ard-2022-223739
Efficacy and safety of emapalumab in macrophage activation syndrome
Abstract
Objectives: Macrophage activation syndrome (MAS) is a severe, life-threatening complication of systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still's disease (AOSD). The objective of this study was to confirm the adequacy of an emapalumab dosing regimen in relation to interferon-γ (IFNγ) activity by assessing efficacy and safety. The efficacy outcome was MAS remission by week 8, based on clinical and laboratory criteria.
Methods: We studied emapalumab, a human anti-IFNγ antibody, administered with background glucocorticoids, in a prospective single-arm trial involving patients who had MAS secondary to sJIA or AOSD and had previously failed high-dose glucocorticoids, with or without anakinra and/or ciclosporin. The study foresaw 4-week treatment that could be shortened or prolonged based on investigator's assessment of response. Patients entered a long-term (12 months) follow-up study.
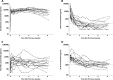
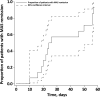
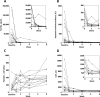
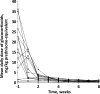
Results: Fourteen patients received emapalumab. All patients completed the trial, entered the long-term follow-up and were alive at the end of follow-up. The investigated dosing regimen, based on an initial loading dose followed by maintenance doses, was appropriate, as shown by rapid neutralisation of IFNγ activity, demonstrated by a prompt decrease in serum C-X-C motif chemokine ligand 9 (CXCL9) levels. By week 8, MAS remission was achieved in 13 of the 14 patients at a median time of 25 days. Viral infections and positive viral tests were observed.
Conclusions: Neutralisation of IFNγ with emapalumab was efficacious in inducing remission of MAS secondary to sJIA or AOSD in patients who had failed high-dose glucocorticoids. Screening for viral infections should be performed, particularly for cytomegalovirus.
Trial registration number: NCT02069899 and NCT03311854.
Keywords: Still's disease, adult-onset; arthritis, juvenile; biological therapy; inflammation; therapeutics.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: FDB: consultant and research grants from AbbVie, Sobi, Pfizer, Roche, Sanofi, Novartis, Novimmune; AAG: consultant for Novartis, AB2 Bio, Novimmune, Sobi; PAB: consultant for Sobi, Novartis, Roche, UCB; CB, MP, GM: none declared; DE: speaker bureau for Sobi; CP: speaker bureau for Sobi; GS: consultant for Novartis, Sobi, Novimmune, AB2 Bio; PQ: consultant for AbbVie, Chugai-Roche, Lilly, Novartis, Pfizer, Sobi and speaker bureau for AbbVie, Chugai-Roche, Lilly, Novartis, Pfizer, Sobi; JA: consultant for Sobi, Novartis, Roche, Pfizer, AbbVie, GSK; CL: consultant for Sobi; RF: previously employed by Sobi; VA: previously employed by Sobi; MB: previously employed by Sobi; PJ: consultant for Sobi; CdM: consultant for Sobi, previously employed by Sobi.
Figures
Comment in
-
A safe and effective treatment for MAS.Nat Rev Rheumatol. 2023 Jun;19(6):326. doi: 10.1038/s41584-023-00974-w. Nat Rev Rheumatol. 2023. PMID: 37147459 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

