Thermophobic Trehalose Glycopolymers as Smart C-Type Lectin Receptor Vaccine Adjuvants
- PMID: 37002787
- PMCID: PMC11212414
- DOI: 10.1002/adhm.202202918
Thermophobic Trehalose Glycopolymers as Smart C-Type Lectin Receptor Vaccine Adjuvants
Abstract
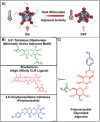
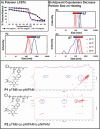
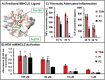
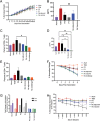
Herein, this work reports the first synthetic vaccine adjuvants that attenuate potency in response to small, 1-2 °C changes in temperature about their lower critical solution temperature (LCST). Adjuvant additives significantly increase vaccine efficacy. However, adjuvants also cause inflammatory side effects, such as pyrexia, which currently limits their use. To address this, a thermophobic vaccine adjuvant engineered to attenuate potency at temperatures correlating to pyrexia is created. Thermophobic adjuvants are synthesized by combining a rationally designed trehalose glycolipid vaccine adjuvant with thermoresponsive poly-N-isoporpylacrylamide (NIPAM) via reversible addition fragmentation chain transfer (RAFT) polymerization. The resulting thermophobic adjuvants exhibit LCSTs near 37 °C, and self-assembled into nanoparticles with temperature-dependent sizes (90-270 nm). Thermophobic adjuvants activate HEK-mMINCLE and other innate immune cell lines as well as primary mouse bone marrow derived dendritic cells (BMDCs) and bone marrow derived macrophages (BMDMs). Inflammatory cytokine production is attenuated under conditions mimicking pyrexia (above the LCST) relative to homeostasis (37 °C) or below the LCST. This thermophobic behavior correlated with decreased adjuvant Rg is observed by DLS, as well as glycolipid-NIPAM shielding interactions are observed by NOESY-NMR. In vivo, thermophobic adjuvants enhance efficacy of a whole inactivated influenza A/California/04/2009 virus vaccine, by increasing neutralizing antibody titers and CD4+ /44+ /62L+ lung and lymph node central memory T cells, as well as providing better protection from morbidity after viral challenge relative to unadjuvanted control vaccine. Together, these results demonstrate the first adjuvants with potency regulated by temperature. This work envisions that with further investigation, this approach can enhance vaccine efficacy while maintaining safety.
Keywords: C-type lectin receptors; MINCLE; adjuvants; cord factors; glycopolymers; thermoresponsive polymers.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bonam S. R., Partidos C. D., Halmuthur S. K. M., Muller S., Trends Pharmacol. Sci. 2017, 38, 771. - PubMed
-
- VAERS – Data , https://vaers.hhs.gov/data.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

