Emergence and antibody evasion of BQ, BA.2.75 and SARS-CoV-2 recombinant sub-lineages in the face of maturing antibody breadth at the population level
- PMID: 37002990
- PMCID: PMC10060887
- DOI: 10.1016/j.ebiom.2023.104545
Emergence and antibody evasion of BQ, BA.2.75 and SARS-CoV-2 recombinant sub-lineages in the face of maturing antibody breadth at the population level
Abstract
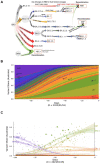
Background: The Omicron era of the COVID-19 pandemic commenced at the beginning of 2022 and whilst it started with primarily BA.1, it was latter dominated by BA.2 and the related sub-lineage BA.5. Following resolution of the global BA.5 wave, a diverse grouping of Omicron sub-lineages emerged derived from BA.2, BA.5 and recombinants thereof. Whilst emerging from distinct lineages, all shared similar changes in the Spike glycoprotein affording them an outgrowth advantage through evasion of neutralising antibodies.
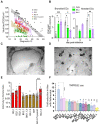
Methods: Over the course of 2022, we monitored the potency and breadth of antibody neutralization responses to many emerging variants in the Australian community at three levels: (i) we tracked over 420,000 U.S. plasma donors over time through various vaccine booster roll outs and Omicron waves using sequentially collected IgG pools; (ii) we mapped the antibody response in individuals using blood from stringently curated vaccine and convalescent cohorts. (iii) finally we determine the in vitro efficacy of clinically approved therapies Evusheld and Sotrovimab.
Findings: In pooled IgG samples, we observed the maturation of neutralization breadth to Omicron variants over time through continuing vaccine and infection waves. Importantly, in many cases, we observed increased antibody breadth to variants that were yet to be in circulation. Determination of viral neutralization at the cohort level supported equivalent coverage across prior and emerging variants with isolates BQ.1.1, XBB.1, BR.2.1 and XBF the most evasive. Further, these emerging variants were resistant to Evusheld, whilst increasing neutralization resistance to Sotrovimab was restricted to BQ.1.1 and XBF. We conclude at this current point in time that dominant variants can evade antibodies at levels equivalent to their most evasive lineage counterparts but sustain an entry phenotype that continues to promote an additional outgrowth advantage. In Australia, BR.2.1 and XBF share this phenotype and, in contrast to global variants, are uniquely dominant in this region in the later months of 2022.
Interpretation: Whilst the appearance of a diverse range of omicron lineages has led to primary or partial resistance to clinically approved monoclonal antibodies, the maturation of the antibody response across both cohorts and a large donor pools importantly observes increasing breadth in the antibody neutralisation responses over time with a trajectory that covers both current and known emerging variants.
Funding: This work was primarily supported by Australian Medical Foundation research grants MRF2005760 (SGT, GM & WDR), Medical Research Future Fund Antiviral Development Call grant (WDR), the New South Wales Health COVID-19 Research Grants Round 2 (SGT & FB) and the NSW Vaccine Infection and Immunology Collaborative (VIIM) (ALC). Variant modeling was supported by funding from SciLifeLab's Pandemic Laboratory Preparedness program to B.M. (VC-2022-0028) and by the European Union's Horizon 2020 research and innovation programme under grant agreement no. 101003653 (CoroNAb) to B.M.
Keywords: Covid-19; Evusheld; Neutralising antibodies; SARS-CoV-2; Sotrovimab; TMPRSS2; Variants.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Funding bodies did not contribute to study design, data collection, data analysis or writing of the manuscript. Study design, data collection, data analysis and data interpretation was performed by the listed authors. Writing and review was performed by the listed authors. A.L.C. Participated on the AstraZeneca COVID-19 Advisory Board (2021), Seqirus COVID Advisory Board (2020) & CSL Seqirus APAC Advisory Council (2022). N.R., T.B., S.A.C., S.M., and T.H. are employees and shareholders of CSL LTD, a manufacturer of the polyclonal immunoglobulin preparations used as a reagent within the study.
Figures



References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

