MiR-27b attenuates mitochondrial oxidative stress and inflammation in endothelial cells
- PMID: 37003179
- PMCID: PMC10090437
- DOI: 10.1016/j.redox.2023.102681
MiR-27b attenuates mitochondrial oxidative stress and inflammation in endothelial cells
Abstract
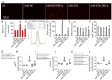
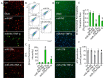
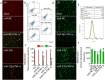
MiR-27b is highly expressed in endothelial cells (EC) but its function in this context is poorly characterized. This study aims to investigate the effect of miR-27b on inflammatory pathways, cell cycle, apoptosis, and mitochondrial oxidative imbalances in immortalized human aortic endothelial cells (teloHAEC), human umbilical vein endothelial cells (HUVEC), and human coronary artery endothelial cells (HCAEC) exposed to TNF-α. Treatment with TNF-α downregulates the expression of miR-27b in all EC lines, promotes the activation of inflammatory pathways, induces mitochondrial alteration and reactive oxygen species accumulation, fostering the induction of intrinsic apoptosis. Moreover, miR-27b mimic counteracts the TNF-α-related cytotoxicity and inflammation, as well as cell cycle arrest and caspase-3-dependent apoptosis, restoring mitochondria redox state, function, and membrane polarization. Mechanistically, hsa-miR-27b-3p targets the 3'untranslated regions of FOXO1 mRNA to downregulate its expression, blunting the activation of the Akt/FOXO1 pathway. Here, we show that miR-27b is involved in the regulation of a broad range of functionally intertwined phenomena in EC, suggesting its key role in mitigating mithochondrial oxidative stress and inflammation, most likely through targeting of FOXO1. Overall, results reveal for the first time that miR-27b could represent a possible target for future therapies aimed at improving endothelial health.
Keywords: Apoptosis; Endothelial dysfunction; Inflammation; Mitochondria; hsa-miR-27b-3p.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures


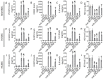


References
-
- Sardu C., Paolisso P., Sacra C., Mauro C., Minicucci F., Portoghese M., Rizzo M.R., Barbieri M., Sasso F.C., D'Onofrio N., Balestrieri M.L., Calabrò P., Paolisso G., Marfella R. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: the CODYCE multicenter prospective study. Diabetes Care. 2019;42:1946–1955. doi: 10.2337/dc18-2356. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

