BISCIT: Biliary interventions in critically ill patients with secondary sclerosing cholangitis-a study protocol for a multicenter, randomized, controlled parallel group trial
- PMID: 37004078
- PMCID: PMC10067228
- DOI: 10.1186/s13063-023-07260-w
BISCIT: Biliary interventions in critically ill patients with secondary sclerosing cholangitis-a study protocol for a multicenter, randomized, controlled parallel group trial
Abstract
Background: Progress of cholangitis to cholangiosepsis is a frequent observation in patients with secondary sclerosing cholangitis in critically ill patients (SSC-CIP). Adequate biliary drainage may reduce episodes of cholangiosepsis and therefore stabilize liver function and improve survival. The primary objective of the BISCIT study is to demonstrate that scheduled biliary interventions will reduce incidence of cholangiosepsis, liver transplantation, or death in patients with SSC-CIP.
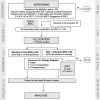
Methods: A total of 104 patients will be randomized at ten study sites. Patients with SSC-CIP, confirmed by endoscopic retrograde cholangiography (ERC), will be randomized 1:1 either in the intervention group which will be treated with scheduled biliary interventions (i.e., therapeutic ERC) every 8 weeks for 6 months or in the control group which will receive standard of care. The randomization will be stratified by center. The composite primary efficacy endpoint is defined as (1) occurrence of death, (2) necessity of liver transplantation, or (3) occurrence of cholangiosepsis within 6 months following randomization.
Discussion: Prospective evaluation of endoscopic treatment procedures is urgently needed to establish an evidence-based therapeutic treatment algorithm in SSC-CIP. A positive trial result could change the current standard of care for patients with SSC-CIP. The results of this study will be disseminated through presentations at international congresses, workshops, and peer-reviewed publications.
Trial registration: The trial was registered at ClinicalTrials.gov (NCT05396755, date of registration: May 31, 2022, last update: May 31, 2022).
Keywords: Biliary interventions; Cholangiosepsis; Critical ill patients; Endoscopic retrograde cholangiography; Randomized-controlled trial; Secondary sclerosing cholangitis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical