A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases
- PMID: 37004361
- PMCID: PMC10111960
- DOI: 10.1016/j.ebiom.2023.104539
A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases
Abstract
Background: The cytokine interleukin-2 (IL-2) can stimulate both effector immune cells and regulatory T (Treg) cells. The ability of selectively engaging either of these effects has spurred interest in using IL-2 for immunotherapy of cancer and autoimmune diseases. Thus, numerous IL-2-based biologic agents with improved bias or delivery towards effector immune cells or Treg cells have been developed. This study systematically reviews clinical results of improved IL-2-based compounds.
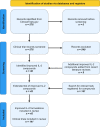
Methods: We searched the ClinicalTrials.gov database for registered trials using improved IL-2-based agents and different databases for available results of these studies.
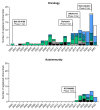
Findings: From 576 registered clinical trials we extracted 36 studies on different improved IL-2-based compounds. Adding another nine agents reported in recent literature reviews and based on our knowledge totalled in 45 compounds. A secondary search for registered clinical trials of each of these 45 compounds resulted in 141 clinical trials included in this review, with 41 trials reporting results.
Interpretation: So far, none of the improved IL-2-based compounds has gained regulatory approval for the treatment of cancer or autoimmune diseases. NKTR-214 is the only compound completing phase 3 studies. The PIVOT IO-001 trial testing the combination of NKTR-214 plus Pembrolizumab compared to Pembrolizumab monotherapy in metastatic melanoma missed its primary endpoints. Also the PIVOT-09 study, combining NKTR-214 with Nivolumab compared to Sunitinib or Cabozantinib in advanced renal cell carcinoma, missed its primary endpoint. Trials in autoimmune diseases are currently in early stages, thus not allowing definite conclusions on efficacy.
Funding: This work was supported by public funding agencies.
Keywords: Autoimmune disease; Cancer; IL-2; Immunotherapy; Interleukin 2; Systematic review.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests MR, UK, and OB hold patents on improved IL-2-based compounds and OB is a shareholder of Anaveon AG. MR discloses paid consulting activities for Urogen. DS states no competing interests related to this work.
Figures

References
-
- Boyman O., Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190. - PubMed
-
- Yu A., Snowhite I., Vendrame F., et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes. 2015;64(6):2172–2183. - PubMed
-
- Hernandez R., Poder J., LaPorte K.M., Malek T.R. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. 2022;22(10):614–628. - PubMed
-
- Donohue J.H., Rosenberg S.A. The fate of interleukin-2 after in vivo administration. J Immunol. 1983;130(5):2203–2208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

